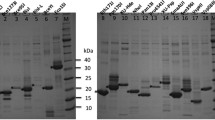
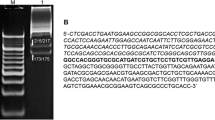
Abstract
AvaI andBsoBI restriction endonucleases are isoschizomers which recognize the symmetric sequence 5′CYCGRG3′ and cleave between the first C and second Y to generate a four-base 5′ extension. TheAvaI restriction endonuclease gene (avaIR) and methylase gene (avaIM) were cloned intoEscherichia coli by the methylase selection method. TheBsoBI restriction endonuclease gene (bsoBIR) and part of theBsoBI methylase gene (bsoBIM) were cloned by the “endo-blue” method (SOS induction assay), and the remainder ofbsoBIM was cloned by inverse PCR. The nucleotide sequences of the two restriction-modification (RM) systems were determined. Comparisons of the predicted amino acid sequences indicated thatAvaI andBsoBI endonucleases share 55% identity, whereas the two methylases share 41% identity. Although the two systems show similarity in protein sequence, their gene organization differs. TheavaIM gene precedesavaIR in theAvaI RM system, while thebsoBIR gene is located upstream ofbsoBIM in theBsoBI RM system. BothAvaI andBsoBI methylases contain motifs conserved among the N4 cytosine methylases.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Athanasiadis A, Blassi M, Kotsifaki D, Tucker PA, Wilson KS, Kokkinidis M (1994) Crystal structure ofPvuII endonuclease reveals extensive structural homologies toEcoRV. Struct Biol 1:469–475
Barany F, Danzitz M, Zebala J, Mayer A (1992) Cloning and sequencing of genes encoding theTthHB8I restriction and modification enzymes: comparison with the isoschizomericTaqI enzymes. Gene 112:3–12
Bickle TA, Kruger DH (1993) Biology of DNA restriction. Microbiol Rev 57:434–450
Cheng X, Balendiran K, Schildkraut I, Anderson JE (1994) Structure ofPvuII endonuclease with cognate DNA. EMBO J 13:3927–3935
Düsterhöft A, Erdmann D, and Kröger M (1991) Isolation and genetic structure of theAvaII isoschizomeric restriction-modification systemHgiBI fromHerpetosiphon giganteus Hpg5: M.HgiBI reveals high homology to M.BanI. Nucleic Acids Res 19:3207–3211
Fomenkov A, Xiao J-p, Dila D, Raleigh E, Xu S-y (1994) The ‘endo-blue method’ for direct cloning of restriction endonuclease genes inE. coli. Nucleic Acids Res 22:2399–2403
Heitman J (1993) On the origins, structures and functions of restriction-modification enzymes. Plenum Press, New York
Heitman J, Model P (1991) SOS induction as an in vivo assay of enzyme-DNA interactions. Gene 103:1–9
Herdman M (1982) Evolution and genetic properties of cyanobacterial genomes. University of California Press, Berkeley
Hughes SG, Murray K (1980) The nucleotide sequences recognized by endonucleasesAvaI andAvaII fromAnabaena variabilis. Biochem J 185:65–75
Kapfer W, Walter J, Trautner TA (1991) Cloning, characterization and evolution of theBsuFI restriction endonuclease gene ofBacillus subtilis and purification of the enzyme. Nucleic Acids Res 19:6457–6463
Kenyon CJ, Walker GC (1980) DNA-damaging agents stimulate expression at specific loci inEscherichia coli. Proc Natl Acad Sci USA 77:2819–2823
Kim YC, Grable JC, Love R, Greene PJ, Rosenberg JM (1990) Refinement ofEcoRI endonuclease crystal structure: a revised protein chain tracing. Science 249:1307–1309
Kita K, Suisha M, Kotani H, Yanase H, Kato N (1992) Cloning and sequence analysis of theStsI restriction-modification gene: presence of homology toFokI restriction-modification enzymes. Nucleic Acids Res 20:4167–4172
Lacks SA, Mannarelli BM, Springhorn SS, Greenberg B (1986) Genetic basis of the complementaryDpnI andDpnII restriction systems ofS. pneumoniae: an intercellular cassette mechanism. Cell 46:993–1000
Lunnen KD, Barsomian JM, Camp RR, Card CO, Chen SZ, Croft R, Looney MC, Meda MM, Moran LS, Nwankwo DO, Slatko BE, VanCott EM, Wilson GG (1988) Cloning type-II restriction and modification genes. Gene 74:25–32
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Mann MB, Rao RN, Smith HO (1978) Cloning of restriction and modification genes inE. coli: theHhaII system fromHaemophilus haemolyticus. Gene 3:97–112
Matsudaira P (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262:10035–10038
Newman M, Strzelecka T, Dorner LF, Schildkraut I, Aggarwal AK (1994) Structure of restriction endonucleaseBamHI and its relationship toEcoRI. Nature 368:660–664
Newman M, Strzelecka T, Dorner LF, Schildkraut I, Aggarwal AK (1995) Structure ofBamHI endonuclease bound to DNA: Partial folding and unfolding on DNA binding. Science 269:656–663
Moyer-Weidner M, Trautner TA (1993) Methylation of DNA in prokaryotes. Birkhäuser, Basel
Nwankwo DO, Wilson GG (1988) Cloning and expression of theMspI restriction and modification genes. Gene 64:1–8
Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623
Piekarowicz A, Yuan R, Stein DC (1991) A new method for the rapid identification of genes encoding restriction and modification enzymes. Nucleic Acids Res 19:1831–1835
Posfai J, Bhagwat AS, Posfai G, Roberts RJ (1989) Predictive motifs derived from cytosine methyltransferases. Nucleic Acids Res 17:2421–2435
Roberts RJ, Halford SE (1993) Type II restriction endonucleases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Roberts RJ, Macelis D (1996) REBASE-restriction enzymes and methylases. Nucleic Acids Res 24:223–235
Seeber S, Kessler C, Götz (1990) Cloning, expression and characterization of theSau3AI restriction and modification genes inStaphylococcus carnosus TM300. Gene 94:37–43
Stephenson FH, Ballard BT, Boyer HW, Rosenberg JM, Greene PJ (1989) Comparison of the nucleotide and amino acid sequences of theRsrI andEcoRI restriction endonucleases. Gene 85:1–13
Szomolanyi E, Kiss A, Venetianer P (1980) Cloning the modification methylase gene ofBacillus sphaericus R inEscherichia coli. Gene 10:219–225
Waite-Rees PA, Keating CJ, Moran LS, Slatko BE, Hornstra LJ, Benner JS (1991) Characterization and expression of theEscherichia coli Mrr restriction system. J Bacteriol 173:5207–5219
Wilson G (1991) Organization of restriction-modification systems. Nucleic Acids Res 19:2539–2566
Wilson GG, Murray NE (1991) Restriction and modification systems. Annu Rev Genet 25:585–627
Winkler FK (1992) Structure and function of restriction endonucleases. Curr Opin Struct Biol 2:93–99
Winkler FK, Banner DW, Tsernoglou D, Brown RS, Heathman SP, Bryan RK, Martin PD, Petratos K, Wilson KS (1993) The crystal structure ofEcoRV endonuclease and of its complexes with cognate and non-cognate DNA fragments. EMBO J 12:1781–1795
Wolf J, Sharp RJ (1981) Taxonomic and related aspects of thermophiles within the genusBacillus. Academic Press, London, pp 251–296
Xu G-L, Kapfer W, Walter J, Trautner TA (1992)BsuBI-an isospecific restriction and modification system ofPstI: characterization of theBsuBI genes and enzymes. Nucleic Acids Res 20:6517–6523
Author information
Authors and Affiliations
Additional information
Communicated by W. Arber
Rights and permissions
About this article
Cite this article
Ruan, H., Lunnen, K.D., Scott, M.E. et al. Cloning and sequence comparison ofAvaI andBsoBI restriction-modification systems. Molec. Gen. Genet. 252, 695–699 (1996). https://doi.org/10.1007/BF02173975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02173975