Summary
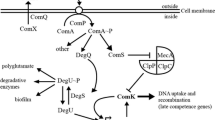
Using the transformation-deficient mutant M465, which was previously isolated by means of insertional mutagenesis with plasmid pHV60, a transcription unit comL required for genetic competence of Bacillus subtilis was identified. A chromosomal DNA fragment flanking the inserted pHV60 was isolated and used to screen two different libraries of B. subtilis DNA in phage lambda EMBL4 and lambda EMBL12, respectively. With the aid of six recombinant phages that hybridize with this chromosomal fragment a restriction map of about 23 kb of B. subtilis chromosomal DNA was constructed. Using small adjoining pieces of this chromosomal DNA in Campbell integrations, the size of the transcription unit involved in competence development could be delimited to about 15 kb. By insertion of a promoterless lacZ gene into comL, the transcriptional regulation of comL was analysed and epistatic interactions among various other com genes were determined. The results of these experiments indicated that comL is optimally expressed in glucose-based minimal medium when the culture enters the stationary phase of growth and that the expression of late competence genes is dependent on previous transcription of comL, which in turn is dependent on the gene products of comA and comB.
Similar content being viewed by others
References
Albano M, Hahn J, Dubnau D (1987) Expression of competence genes in Bacillus subtilis J Bacteriol 169:3110–3117
Albano M, Dubnau D (1989) Cloning and characterization of a cluster of linked Bacillus subtilis late competence mutants. J Bacteriol 171:5376–5385
Biswal N, Kleinschmidt AK, Spatz HC, Trautner TA (1967) Physical properties of the DNA of the bacteriophage SP50. Mol Gen Genet 100:39–55
Bron S, Venema G (1972) Ultraviolet inactivation and excision repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res 15:1–10
Casadaban MJ, Conen SN (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol 138:179–207
Chambers SP, Prior S, Barstow DB, Minton NP (1988) The pMTL series of cloning vectors: I. Improved polylinker regions to facilitate the generation of sonicated DNA for nucleotide sequencing. Gene 68:139–149
Chomczynski P, Qasba PK (1984) Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun 122:340–344
Dubnau D (1989) The competence regulon of Bacillus subtilis. In: Smith I, Slepecky R, Setlow P (eds) Regulation of prokaryotic development. American Society for Microbiology, Washington DC, pp 147–166
Dubnau D, Roggiani M (1990) Growth medium-independent genetic competence mutants of Bacillus subtilis. J Bacteriol 172:4048–4055
Dubnau E, Weir J, Nair G, Carter III, HL, Moran Jr, CP, Smith I (1988) Bacillus sporulation gene spoOH codes for σ30 (σH). J Bacteriol 170:1054–1062
Frischauf AM, Lehrach H, Poustka A, Murray N (1983) Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170:827–842
Gaur NK, Dubnau E, Smith I (1986) Characterization of a cloned Bacillus subtilis gene which inhibits sporulation in multiple copies. J Bacteriol 168:860–869
Guillen N, Weinranch Y, Dubnau D (1989) Cloning and characterization of the regulatory Bacillus subtilis competence genes, comA and comB. J Bacteriol 171:5354–5361
Hadden C, Nester EW (1968) Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol 95:876–885
Haseltine Cahn F, Fox MS (1968) Fractionation of transformable bacteria from competent cultures of Bacillus subtilis on renografin gradients. J Bacteriol 95:867–875
Henner DJ, Yang M, Ferrari E (1988) Localization of Bacilus subtilis sacU (HY) mutations to two linked genes with similarities to the conserved procaryotic family of two-component signaling systems. J Bacteriol 170:5102–5109
Hoch JA, Trach K, Kawamura F, Saito H (1985) Identification of the transcriptional suppressor sof-1 as an alteration in the spoOA protein. J Bacteriol 161:552–555
Horinouchi S, Weisblum B (1982) Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol 150:8I5–825
Ish-Horowicz D, Burke FJ (1981) Rapid and efficient cosmid cloning. Nucleic Acids Res 9:2989–2999
Jaacks KJ, Healy J, Losick R, Grossman AD (1989) Identification and characterization of genes controlled by the sporulationregulation gene spoOH in Bacillus subtilis. J Bacteriol 171:4121–4129
Kiel JAKW, Vossen JPMJ, Venema G (1987) A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid seqregation. Mol Gen Genet 207:294–301
Kunst F, Debarbouille M, Msadek T, Young M, Mauel C, Karamata D, Klier A, Rapoport G, Dedonder R (1988) Deduced polypeptides encoded by the Bacillus subtilis sacU locus share homology with two-component sensor-regulator systems. J Bacteriol 170:5093–5101
Mandel M, Higa A (1970) Calcium-dependent bacteriophage DNA infection. J Mol Biol 53:159–162
Maniatis T, Fritsch EF, Sambrook F (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Marahiel MA, Krause M, Sharpeid H (1985) Cloning of the tyrocidine synthetase I gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet 201:231–236
Messing J (1979) A multipurpose cloning system based on the single stranded DNA bacteriophage M13. Recombinant DNA Technical Bulletin, NIH Publication, No. 79–99, 2, No. 2, pp 43–48
Miller JH (1982) Experiments in molecular genetics Cold Spring Harbor Laboratory, Cold Spring Harbor New York
Mittenhuber G, Weckermann R, Marahiel MA (1989) gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol 171:4881–4887
Msadek T, Kunst F, Henner D, Klier A, Rapoport G, Dedonder R (1990) The signal transduction pathway controlling the synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analyses of mutations in degS and degU. J Bacteriol 170:824–834
Nakano MM, Zuber P (1989) Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol 171:5347–5353
Natt E, Scherer G (1986) EMBL12, a new lambda replacement vector with sites for Sall, XbaI, BamH1, Sst1 and EcoRI. Nucleic Acids Res 14:7128
Perego M, Spiegelman GB, Hoch JA (1988) Structure of the gene for the transition state regulator abrB: regulator synthesis is controlled by the spoOA sporulation gene in Bacillus subtilis. Mol Microbiol 2:689–699
Perkins JB, Youngman PJ (1986) Construction and properties of T017-lac, a transposon derivative that mediates transcriptional gene fusions in Bacillus subtilis. Proc Natl Acad Sci USA 83:140–144
Rigby PWJ, Dieckman M, Rhodes C, Berg P (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113:237–251
Roggiani M, Hahn J, Dubnau D (1990) Suppression of early competence mutations in Bacillus subtilis by mec mutations. J Bacteriol 172:4056–4063
Rosenthal AL, Lacks SA (1977) Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem 80:76–90
Sadaie Y, Kada T (1983) Formation of competent Bacillus subtilis cells. J Bacteriol 153:813–821
Schaeffer P, Millet I, Aubert J (1965) Catabolite repression of bacterial sporulation. Proc Natl Acad Sci USA 54:704–761
Spizizen J (1958) Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA 44:1072–1078
Stock JB, Ninfa AJ, Stock AM (1989) Protein phosphorylation and the regulation of adaptive responses in bacteria. Microbiol Rev 53:450–490
Tanaka T, Kawata M (1988) Cloning and characterization of Bacillus subtilis iep, which has positive and negative effects on production of extracellular proteases. J Bacteriol 170:3593–3600
Venema G, Pritchard RH, Venema-Schröder T (1965) Fate of transforming deoxyribonucleic acid in Bacillus subtilis. J Bacteriol 89:1250–1255
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Vosman B, Kooistra J, Olijve J, Venema G (1986) Integration of vector-containing Bacillus subtilis chromosomal DNA by a Campbell-like mechanism. Mol Gen Genet 204:524–531
Vosman B, Kooistra J, Olijve J, Venema G (1987) Cloning in Escherichia coli of the gene specifying the DNA-entry nuclease of Bacillus subtilis. Gene 52:175–183
Vosman B, Kuiken G, Kooistra J, Venema G (1988) Transformation in Bacillus subtilis: Involvement of the 17-kilodalton DNAentry nuclease and the competence specific 18-kilodalton protein. J Bacteriol 170:3703–3710
Weckermann R, Fûrbass R, Marahiel MA (1988) Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase I from Bacillus brevis. nucleic Acids Res 16:11841
Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D (1990) A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev 4:860–872
Weinstock GM, Berman ML, Silhavy TJ (1983) Chimeric genes with β-galactosidase. In: Papas TS, Rosenberg M, Chirikjian JG (eds) Gene amplification and analysis III. Elsevier, North Holland, New York, pp 27–64
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mpl8 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Additional information
Communicated by H. Böhme
Rights and permissions
About this article
Cite this article
van Sinderen, D., Withoff, S., Boels, H. et al. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis . Molec. Gen. Genet. 224, 396–404 (1990). https://doi.org/10.1007/BF00262434
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00262434