Summary
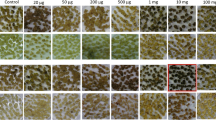
A protoplast mutagenesis and cell selection system was used for the isolation of streptomycin resistant Lycopersicon peruvianum colonies. Protoplasts were treated with the mutagen N-nitroso-methylurea and could be regenerated into fertile plants, carrying the streptomycin resistant character. Several classes of streptomycin resistance could be distinguished. Reciprocal crosses between streptomycin resistant and sensitive plants showed a non-Mendelian transmission of the resistance trait. Streptomycin resistance is the first selectable and maternally inherited cell organelle marker described in tomato.
Similar content being viewed by others
References
Barlett SG, Harris EH, Grabowy CT (1979) Ribosomal subunits affected by antibiotic resistance mutations at seven chloroplast loci in Chlamydomonas reinhardtii. Mol Gen Genet 176:199–208
Binding H, Nehls R (1977) Regeneration of isolated protoplasts to plants in Solanum dulcamara L. Z Pflanzenphysiol 85:279–280
Binding H, Binding K, Straub J (1970) Selektion in Gewebekulturen mit haploiden Zellen. Naturwissenschaften 57:138–139
Caboche M, Muller JF (1980) Use of a medium allowing low cell density growth for in vitro selection experiments: isolation of valine resistant clones from nitrosoguanidine-mutagenized cells and gamma-irradiated tobacco plants. In: Sala F, Parisi B, Cella R, Ciferri O (eds) Plant cell cultures: results and perspectives. Elsevier, Amsterdam, pp 133–138
Cseplo A, Maliga P (1982) Lincomycin resistance, a new type of maternally inherited mutation in Nicotiana plumbaginifolia. Curr Genet 6:105–109
Cseplo A, Maliga P (1984) Large scale isolation of maternally inherited lincomycin resistance mutations in diploid Nicotiana plumbaginifolia protoplast cultures. Mol Gen Genet 196:407–412
Cseplo A, Etzold T, Schell J, Schreier PH (1988) Point mutations in the 23 S rRNA genes of four lincomycin resistant Nicotiana plumbaginifolia mutants could provide new selectable markers for chloroplast transformation. Mol Gen Genet 214:295–299
Davis BD, Tai P-C, Wallace BJ (1974) Complex interactions of antibiotics with the ribosome. In: Nomura M, Tissieres A, Lengyel P (eds) Ribosomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 771–791
Dix PJ, Joo F, Maliga P (1977) A cell line of Nicotiana sylvestris with resistance to kanamycin and streptomycin. Mol Gen Genet 157:285–290
Edwards D (1980) Antimicrobial drug action. Macmillan, London, pp 193–216
Etzold T, Fritz CC, Schell J, Schreier PH (1987) A point mutation in the chloroplast 16 S rRNA gene of a streptomycin resistant Nicotiana tabacum. FEBS Lett 219:343–346
Fluhr R, Aviv D, Galun E, Edelman M (1985) Efficient induction and selection of chloroplast encoded antibiotic resistant mutations in Nicotiana. Proc Natl Acad Sci USA 82:1485–1489
Frearson EM, Power JB, Cocking EC (1973) The isolation, culture and regeneration of Petunia leaf protopiasts. Dev Biol 33:130–137
Fromm H, Edelman M, Aviv D, Galun E (1987) The molecular basis for rRNA-dependent spectinomycin resistance in Nicotiana chloroplasts. EMBO J 6:3233–3237
Fromm H, Galun E, Edelman M (1989) A novel site for streptomycin resistance in the “530 loop” of chloroplast 16S ribosomal RNA. Plant Mol Biol 12:499–505
Funatsu G, Wittmann HG (1972) Ribosomal proteins XXXIII. Location of amino-acid replacements in protein S12 isolated from E. coli mutants resistant to streptomycin. J Mol Biol 68:547–550
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gauthier A, Turmel M, Lemieux C (1988) Mapping of chloroplast mutations conferring resistance to antibiotics in Chlamydomonas: Evidence for a novel site of streptomycin resistance in the small subunit rRNA. Mol Gen Genet 214:192–197
Gillham NW (1965) Induction of chromosomal and nonchromosomal mutations in Chlamydomonas reinhardi with N-methyl-Nnitro-N-nitrosoguadinine. Genetics 52:529–537
Gorini L, Davies J (1968) The effect of streptomycin on ribosomal function. Curr Top Microbiol Immunol 44:100–122
Hagemann R (1982) Induction of plastome mutations by nitrosourea compounds. In: Edelmann M, Hallick RB, Chua N-H (eds) Methods in chloroplast molecular biology. Elsevier, Amsterdam, pp 119–127
Hamill JD, Ahuja PS, Davey MR, Cocking EC (1986) Protoplast derived streptomycin resistant plants of the forage legume Onobrychis viciifolia Scop. (Scinfoin). Plant Cell Rep 5:439–441
Harris EH, Boyton JE, Gillham NW, Tingle CL, Fox SB (1977) Mapping of chloroplast genes involved in chloroplast ribosome biogenesis in Chlamydomonas reinhardtii. Mol Gen Genet 155:249–265
Hille J, Koornneef M, Ramanna MS, Zabel P (1989) Tomato: a crop species amenable to improvement by cellular and molecular methods. Euphytica 42:1–23
Hosticka LP, Hanson MR (1984) Induction of plastid mutations in tomatoes by nitrosomethylurea. J Hered 75:242–246
Koornneef M, van Diepen JAM, Hanhart CJ, Kieboom-de Waart AC, Martinelli L, Schoenmakers HCH, Wijbrandi J (1989) Chromosomal instability of cell and tissue cultures of tomato haploids and diploids. Euphytica 43:179–186
Lee RW, Jones RF (1973) Induction of Mendelian and non-Mendelian streptomycin resistant mutants during synchronous cell cycle of Chlamydomonas reinhardtii. Mol Gen Genet 121:99–108
Lee RW, Gillham NW, Van Winkle KP, Boynton JE (1973) Preferential recovery of uniparental streptomycin resistant mutants from diploid Chlamydomonas reinhardtii. Mol Gen Genet 211:109–116
Lemieux C, Lee RW (1987) Nonreciprocal recombination between alleles of the chloroplast 23S rRNA gene in interspecific Chlamydomonas crosses. Proc Natl Acad Sci USA 84:4166–4170
Lemieux C, Turmel M, Seligy V, Lee RW (1984) Chloroplast DNA recombination in interspecific hybrids of Chlamydomonas: Linkage between a nonmendelian locus for streptomycin resistance and restriction fragments coding for 16 S rRNA. Proc Natl Acad Sci USA 81:1164–1168
Maliga P (1981) Streptomycin resistance is inherited as a recessive mendelian trait in a Nicotiana sylvestris line. Theor Appl Genet 60:1–3
Maliga P, Sz-Breznovits A, Marton L (1973) Streptomycin-resistant plants from callus culture of haploid tobacco. Nature New Biol 244:29–30
Maliga P, Sz-Breznovits A, Marton L (1975) Non-Mendelian streptomycin resistant tobacco mutant with altered chloroplasts and mitochondria. Nature 255:401–402
Maliga P, Sidorov VA, Cseplo A, Menczel L (1981) Induced mutations in advancing in vitro techniques. In: Proceedings of IAEA/FAO International Symposium on induced mutations as a tool in plant research. IAEA, Vienna, pp 339–352
Marton L, Dung TM, Mendel RR, Maliga (1989) Nitrate reductase deficient cell lines from haploid protoplast cultures of Nicotiana plumbaginifolia. Mol Gen Genet 186:301–304
McCabe PF, Timmons AM, Dix PJ (1989) A simple procedure for the isolation of streptomycin resistant plants in Solanaceae. Mol Gen Genet 216:132–137
Melancon P, Lemieux C, Brakier-Gingras L (1988) A mutation in the 530 loop of Escherichia coli 16S ribosomal RNA causes resistance to streptomycin. Nucleic Acids Res 16:9631–9639
Montandon PE (1985) Streptomycin resistance of Euglena gracilis chloroplasts: identification of a point mutation in the 16S rRNA gene in an invariant position. Nucleic Acids Res 13:4299–4310
Montandon PE, Wagner R, Stutz E (1986) E. coli ribosomes with a C912 to U base change in the 16S rRNA are streptomycin resistant. EMBO J 5:3705–3708
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nicolas P (1981) Sensitivity of Euglena gracilis to chloroplast inhibiting antibiotics, and properties of antibiotic-resistant mutants. Plant Sci Lett 22:309–316
Nitsch JP (1951) Experimental androgenesis in Nicotiana. Phytomorphology 19:389–404
O'Connell MA, Hanson MR (1986) Regeneration of somatic hybrid plants formed between Lycopersicon esculentum and Solanum rickii. Theor Appl Genet 72:59–65
O'Connell MA, Hanson MR (1987) Regeneration of somatic hybrid plants formed between Lycopersicon esculentum and L. pennellii. Theor Appl Genet 75:83–89
Ozaki M, Mizushima S, Nomura M (1969) Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature 222:333–339
Richardson KK, Richardson FC, Crosby RM, Swenberg JA, Skopek TR (1987) DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci USA 84:344–348
Rick CM, Yoder JI (1988) Classical and molecular genetics of tomato — highlights and perspectives. Annu Rev Genet 22:281–300
Rick CM, DeVerna JW, Chetelat RT, Stevens MA (1987) Potential contributions of wide crosses to improvement of processing tomatoes. Acta Hortic 200:45–55
Sung ZR (1976) Mutagenesis of cultured plant cells. Genetics 84:51–57
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading
Umiel N (1979) Streptomycin resistance in tobacco: III. A test on germinating seedlings indicates cytoplasmic inheritance in the St-R701 mutant. Z Pflanzenphysiol 92:295–301
Umiel N, Goldner R (1976) Effects of streptomycin on diploid tobacco callus cultures and the isolation of resistant mutants. Protoplasma 89:83–89
Weber G, Lark KG (1980) Quantitative measurement of the ability of different mutagens to induce an inherited change in phenotype to allow maltose utilization in suspension cultures of soybean. Genetics 96:213–222
Wittmann HG, Wittmann-Liebold B (1974) Chemical structure of bacterial ribosomal proteins. In: Nomura M, Tissieres A, Lengyel P (eds) Ribosomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 115–141
Author information
Authors and Affiliations
Additional information
Communicated by R. Hagemann
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Jansen, C.E., Snel, E.A.M., Akerboom, M.J.E. et al. Induction of streptomycin resistance in the wild tomato Lycopersicon peruvianum . Molec. Gen. Genet. 220, 261–268 (1990). https://doi.org/10.1007/BF00260492
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00260492