Abstract
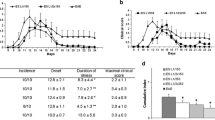
The effect of the adult worms and migrating L1 larvae of Trichinella spiralis on the production of specific IgE antibodies was determined in BCF1 mice. To achieve this, we combined the effect of two anthelminthics: thiabendazole, to produce chemosterilization of adult females, and napthalophos, to expel adult worms from the intestine of infected mice on the desired day. Our results demonstrate that when the natural route of infection is used the production of IgE antibodies is not dependent on the infection dose or the number of migrating L1 larvae, and that both intestinal worms and migrating L1 larvae contribute to the production of reaginic antibodies. In addition to this, an extended period of antigenic stimulation (10–12 days) is required for the induction of a detectable, specific IgE response by adult worms. Finally, our results seem to indicate that although the effects of adult worms and migratory L1 larvae on the IgE production are not additive, the presence of adult worms in the intestine of mice may stimulate a secondary exposure to common antigens released by the migrating L1 larvae.
Similar content being viewed by others
References
Almond NM, McLaren DJ, Parkhouse RME (1986) A comparison of the surface and secretions of Trichinella pseudospiralis and Trichinella spiralis. Parasitology 93:163–176
Bartelmez SH, Dodge WH, Bass DA (1982) Antigen-mediated release of eosinophil growth stimulating factor from Trichinella spiralis sensitized spleen cells: a comparison of Trichinella spiralis stage-specific antigen preparations. Immunology 45:605–611
Campbell WC, Cuckler AC (1964) Effect of thiabendazole upon the enteral and parenteral phases of trichinosis in mice. J Parasitol 50:481–488
Dessein AJ, Parker WL, James SL, David JR (1981) IgE antibody and resistance to infection: I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med 153:423–436
Gabriel BW, Justus DF (1979) Quantitation of immediate and delayed hypersensitivity responses in Trichinella-infected mice. Int Arch Allergy Appl Immunol 60:275–285
Ikegami M (1984) Host response in Trichinella spiralis infected mice. The kinetics of mucosal mast cells and IgE antibodies in the small intestine. Jpn J Vet Res 32:95
Ishizaka K, Huff TF, Jardieu P, Moore KW, Martens CL (1985) IgE binding factors. Selective regulation of the IgE response by T cell factors. Int Arch Allergy Appl Immunol 77:13–20
Jarrett EEE (1973) Reaginic antibodies and helminth infections. Vet Rec 93:480–483
Kennedy MW, Wakelin D, Wilson MM (1979) Transplantation of adult Trichinella spiralis between hosts: worm survival and immunological characteristics of the host-parasite relationship. Parasitology 78:121–130
Martinez AR, Cordero del Campillo M, Aller B (1969) Anthelminthic effect of Maretin-Bayer against T. spiralis infections in rats and mice. Wiad Parazytol 15 (5–6):757–758
Ottesen EA, Smith TK, Kirkpatrick CH (1975) Immune response to Trichinella spiralis in the rat. Int Arch Allergy Appl Immunol 49:396–410
Ovary Z (1958) Passive cutaneous anaphylaxis in the mouse. J Immunol 81:355–357
Rivera-Ortiz CI, Nussenzweig R (1976) Trichinella spiralis: Anaphylactic antibody formation and susceptibility in strains of inbred mice. Exp Parasitol 39:7–17
Sanmartín-Durán ML, Santamarina MT, Ubeira FM (1986) Effect of clofibrate and hydrocortisone on intestinal trichinellosis in mice. Vet Parasitol 21:55–60
Spaldonova R, Cerman J, Corba J, Velebny, S (1981) Allergenic reactions of the host after treatment of experimental trichinellosis. In: Kim CW, Ruitenberg EJ, Teppema JS (eds). Trichinollosis. Reedbooks, Chertsey, pp 327–329
Ubeira FM, Leiro J, Santamarína MT, Villa TG, Sanmartin-Durán ML (1987) Immune response to Trichinella epitopes: The antiphosphorylcholine plaque-forming cell response during the biological cycle. Parasitology 94:543–553
Urban JF, Katona IM, Dean DA, Finkleman FD (1984) The cellular IgE response of rodents to infection with Nippostrongylus brasitiensis, Trichinella spiralis and Schistosoma mansoni. Vet Parasitol 14:193–208
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Santamarina, M.T., Leiro, J., Garrido, M.J. et al. The effect of the intestinal worms and migrating L1 larvae of Trichinella spiralis on the production of antiparasitic IgE antibodies. Parasitol Res 74, 581–585 (1988). https://doi.org/10.1007/BF00531638
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00531638