Abstract
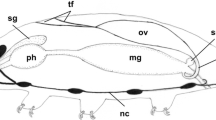
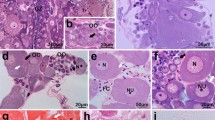
In the sea urchin Hemicentrotus pulcherrimus, the egg-jelly macromolecule, a fucose sulphate glycoconjugate (FSG) that induces the acrosome reaction in spermatozoa, originates from the accessory cells in the ovary. In the present study we examined the seasonal variations in the distribution of FSG in the ovary by immunocytochemistry with a polyclonal antibody. An enzyme-linked immunosorbent assay indicated that FSG was present in supernatants of extracts of ovaries throughout the development of the ovary. However, the immunohistochemical study showed that there are marked seasonal changes in the distribution of FSG in ovaries. The polyclonal antibody reacted strongly with globules of accessory cells before the beginning of the breeding season (August to December). During the breeding season (February to April), the immunohistochemical reaction was found on the surface of oocytes but was weak in the accessory cells. At the ultrastructural level, the antibody reacted with globules of variable density in accessory cells. Intense immunolabelling was observed in the vacuole-like structures of the globules. Sometimes, products of the specific immunocytochemical reaction were found in the Golgi apparatus in these globules. Quantitative examination indicated that FSG was actively produced by the accessory cells from the late non-breeding season to the pre-breeding season. These results suggest that there are marked seasonal variations in the production of FSG by the accessory cells in the sea urchin ovary. These findings also provide new evidence that accessory cells exhibit dynamic changes during the reproductive process in the sea urchin.
Similar content being viewed by others
References
Abe H, Kinoh H, Oikawa T, Suzuki N (1992) The egg-jelly macromolecule, a fucose sulphate glycoconjugate, originates from the accessory cells of the ovary in the sea urchin Hemicentrotus pulcherrimus. Roux's Arch Dev Biol 201:179–189
Chatlynne LG (1969) A histochemical study of oogenesis in the sea urchin, Strongylocentrotus purpuratus. Biol Bull 136:167–184
Christen R, Schackmann RW, Shapiro BM (1983) Interactions between sperm and sea urchin egg jelly. Dev Biol 98:1–14
Collins F, Epel D (1977) The role of calcium ions in the acrosome reaction of sea urchin sperm. Exp Cell Res 106:211–222
Dan JC (1952) Studies on the acrosome. I. Reaction to egg-water and other stimuli. Biol Bull 103:54–66
Dan JC (1954) Studies of the acrosome. III. Effect of calcium deficiency. Biol Bull 107:335–349
Dan JC (1956) The acrosome reaction. Int Rev Cytol 5:365–393
Decker GL, Joseph DB, Lennarz WJ (1976) A study of factors involved in induction of the acrosomal reaction in sperm of the sea urchin, Arbacia punctulata. Dev Biol 53:115–125
Epel D (1978) Mechanisms of activation of sperm and egg during fertilization of sea urchin gametes. Curr Top Dev Biol 12:185–246
Fuji A (1960) Studies on the biology of the sea urchin. I. Superficial and histological gonadal changes in the gametogenic process of two sea urchins, Strongylocentrotus nudus and S. intermedius. Bull Fac Fish Hokkaido Univ 11:1–14
Garbers DL, Watkins HD, Hansbrough JR, Smith A, Misono KS (1982) The amino acid sequence and chemical synthesis of speract and of speract analogues. J Biol Chem 257:2734–2737
Gathers DL, Kopf GS, Tubb DJ, Olson G (1983) Elevation of sperm adenosine 3′:5′-monophosphate concentrations by a fucose-sulphate-rich complex associated with eggs: I. structural characterization. Biol Reprod 29:1211–1220
Hotta K, Hamazaki H, Kurokawa M, Isaka S (1970) Isolation and properties of a new type of sialopolysaccharid-protein complex from the jelly coat of sea urchin eggs. J Biol Chem 245:5434–5440
Isaka S, Hotta K, Kurokawa M (1970) Jelly coat substances of sea urchin eggs. Exp Cell Res 59:37–42
Ishihara K, Oguri K, Taniguchi H (1973) Isolation and characterization of fucose sulfate from jelly coat glycoprotein of sea urchin egg. Biochim Biophys Acta 320:628–634
Jondeung A, Czihak G (1982) Histochemical studies of jelly coat of sea-urchin eggs during oogenesis. Histochemistry 76:123–136
Jondeung A, Czihak G (1983) Histochemical study of globules in accessory cells of sea-urchin ovary: In relation to jelly coat substance. Acta Histochem 73:9–15
Kinsey WH, SeGall GK, Lennarz WJ (1979) The effect of the acrosome reaction on the respiratory activity and fertilizing capacity of echinoid sperm. Dev Biol 71:49–59
Kinsey WH, Rubin JA, Lennarz WJ (1980) Studies on the specificity of sperm binding in echinoderm fertilization. Dev Biol 74:245–250
Kopf GS, Garbers DL (1980) Calcium and a fucose-sulfate-rich polymer regulate sperm cyclic nucleotide metabolism and the acrosome reaction. Biol Reprod 22:1118–1126
Masuda R, Dan JC (1977) Studies on the annual reproductive cycle of the sea urchin and the acid phosphatase activity of relict ova. Biol Bull 153:577–590
Ohtake H (1976) Respiratory behavior of sea-urchin spermatozoa: I. Effect of pH and egg water on the resiratory rate. J Exp Zool 198:303–312
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212
SeGall GK, Lennarz WJ (1979) Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev Biol 71:33–48
SeGall GK, Lennarz WJ (1981) Jelly coat and induction of the acrosome reaction in echinoid sperm. Dev Biol 86:87–93
Shimizu T, Kinoh H, Yamaguchi M, Suzuki N (1990) Purification and characterization of the egg jelly macromolecules, sialoglycoprotein and fucose sulfate glycoconjugate, of the sea urchin Hemicentrotus pulcherrimus. Dev Growth Differ 32:473–487
Summers RG, Hylander BL (1975) Species-specificity of acrosomal reaction and primary gamete binding in echinoids. Exp Cell Res 96:63–68
Summers RG, Hylander BL, Colwin LH, Colwin AL (1975) The functional anatomy of the echinoderm spermatozoon and its interaction with the egg at fertilization. Am Zool 15:523–551
Suzuki N (1989) Sperm-activating peptides from sea urchin egg jelly. In: Scheuer PJ (ed) Bioorganic marine chemistry, vol 3. Springer, Berlin Heidelberg New York, pp 47–70
Suzuki N, Nomura K, Ohtake H, Isaka S (1981) Purification and the primary structure of sperm-activating peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Comm 99:1238–1244
Suzuki N, Kobayashi K, Isaka S (1982) Appearance of sperm activation factors in the ovary of the sea urchin Hemicentrotus pulcherrimus with maturation. Experientia 38:1245–1246
Takashima Y (1976) Ultrastructure of nurse cells and oogenesis in the sea urchin. Gunma Symp Endocrinol 13:105–127
Tilney LG, Hatano S, Ishikawa H, Mooseker MS (1973) The polymerization of actin: Its role in the generation of the acrosomal process of certain echinoderm sperm. J Cell Biol 59:109–126
Tominaga A, Takashima T, Takashima Y (1976) Morphology of the nurse cells in the sea urchin (in Japanese). Acta Anat Nippon 51:127–128
Verhey CA, Moyer FH (1967) Fine structural changes during sea urchin oogenesis. J Exp Zool 164:195–226
Voller A, Bidwell D, Bartless A (1976) Microplate enzyme immunoassay for the immunodiagnosis of virus infection. In: Rose N, Friedman W (eds) Manual of clinical immunology. American Society for Microbiology, Washington DC, pp 506–512
Yamaguchi M, Niwa T, Kurita M, Suzuki N (1988) The participation of speract in the acrosome reaction of Hemicentrotus pulcherrimus. Dev Growth Differ 30:159–167
Yamaguchi M, Kurita M, Suzuki N (1989) Induction of the acrosome reaction of Hemicentrotus pulcherrimus spermatozoa by the egg jelly molecules, fucose-rich glycoconjugate and sperm-activating peptide I. Dev Growth Differ 31:233–239
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abe, H., Kinoh, H. & Suzuki, N. Seasonal variations in the production of the egg-jelly macromolecule, a fucose sulphate glycoconjugate, by the accessory cells in the ovary of the sea urchin Hemicentrotus pulcherrimus . Roux's Arch Dev Biol 203, 402–410 (1994). https://doi.org/10.1007/BF00188689
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00188689