Summary
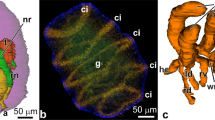
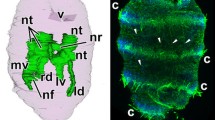
The presumptive ectoderm (pE) ofCynops gastrulae was artificially mesodermalized by contact with teleost swimbladder. The newly mesodermalized ectoderm (mE) acquired the capacity for neural induction (Suzuki et al. 1986a). SEM observations revealed that the mE cells altered their cellular profiles immediately after mesodermalization. The characteristics of the cell surface and the cell architecture became similar to those of invaginated mesoderm cells. There were distinct differences in the cellular contact between mE—pE and pE—pE combinations. The mE-pE combinations kept close contact at their interior surfaces, while the pE—pE combinations did not keep contact. Both TEM and SEM observations also indicated that there were tight contacts between mE and pE cells. These findings suggest that neural-inducing activity of the newly mesodermalized ectoderm cells is coupled with acquisition of cellular affinity toward the interior surface of competent ectoderm cells, and probably requires close cell contacts.
Similar content being viewed by others
References
Asashima M, Grunz H (1983) Effects of inducers on inner and outer gastrula ectoderm layers ofXenopus laevis. Differentiation 23:206–212
Bellairs R, Curtis ASG, Sanders EJ (1978) Cell adhesiveness and embryonic differentiation. J Embryol Exp Morphol 46:207–213
Dixon JE, Kintner CR (1989) Cellular contacts required for neural induction inXenopus embryos: evidence for two signals. Development 106:749–757
Duprat AM, Gualandris L, Rougé P (1982) Neural induction and the structure of the target cell surface. J Embryol Exp Morphol 70:171–187
Duprat AM, Kan P, Gualandris L, Foulquier F, Marty J, Weber M (1985) Neural induction: embryonic determination elicits full expression of specific neuronal traits. J Embryol Exp Morphol 89:167–183
Edelman GM (1984) Cell adhesion and morphogenesis: the regulator hypothesis. Proc Natl Acad Sci USA 81:1460–1464
Fontana DR, Price PL (1989) Cell-cell contact elicits cAMP section and alters cAMP signaling inDicytostelium discoideum. Differentiation. 41:184–192
Grunz H, Staubach J (1979a) Cell contacts between chorda-mesoderm and the overlying neuroectoderm (presumptive central nervous system) during the period of primary embryonic induction in amphibians. Differentiation 14:59–65
Grunz H, Staubach J (1979b) Changes of the cell surface charge of amphibian ectoderm after induction. Rouxs Arch Dev Biol 186:77–80
Grunz H, Tacke L (1989) Neural differentiation ofXenopus laevis ectoderm takes place after disaggegation and delayed reaggregation without inducer. Cell Differ 28:211–218
Gualandris L, Duprat AM (1981) A rapid experimental method to study primary embryonic induction. Differentiation 20:270–273
Gualandris L, Duprat AM (1987) Structural alteration of the target plasma membrane affects reception but not expression of the neural inductitve signal. Cell Differ 20:147–152
Gualandris L, Rougé P, Duprat AM (1983) Membrane changes in neural target cells studied with fluorescent lectin probes. J Embryol Exp Morphol 77:183–200
Holtfreter J (1947) Neural induction in explants which have passed through a sublethal cytolysis. J Exp Zool 106:197–222
Ito S, Ikematsu Y (1980) Inter- and intratissue communication during amphibian development. Dev Growth Differ 22:247–254
Kaneda T, Suzuki AS (1983) Studies on the formation and state of determination of the trunk organizer in the newt,Cynops pyrrhogaster: IV. The association of the neural-inducing activity with the mesodermalization of the trunk organizer. Rouxs Arch Dev Biol 192:8–12
Kawakami I (1976) Fish swimbladder: an excellent mesodermal inductor in primary embryonic induction. J Embryol Exp Morphol 36:315–320
Keller RK (1987) Cell behavior during active cell rearrangement: evidence and speculations. J Cell Sci Suppl 8:369–393
Komazaki S (1982) Morphological changes of the inner surface of the blastocoelic wall before and during gastrulation in the newt,Cynops pyrrhogaster. Dev Growth Differ 24:491–499
Matsuda M, Oya T (1977) Induced oviposition in the newt,Cynops pyrrhogaster by subcutaneous injection of human chorionic gonadotropin. Zool Mag (Tokyo) 86:44–47
Monroy A, Baccetti B, Denis-Donini S (1976) Morphological changes of the surface of the egg ofXenopus laevis in the course of development: III. Scanning electron microscopy of gastrulation. Dev Biol 49:250–259
Okada YoK, Ichikawa M (1947) Normal table ofTriturus pyrrhogaster. Jpn J Exp Morphol 3:1–6
Otte AP, Koster CH, Snoke GT, Durston SJ (1988) Protein kinase C mediates neural induction inXenopus laevis. Nature 334:618–620
Saxén L (1961) Transfilter neural induction of amphibian ectoderm. Dev Biol 3:140–152
Smith JC, Symes K, Hynes RO, DeSimone D (1990) Mesoderm induction and the control of gastrulation inXenopus laevis: the roles of fibronectin and integrins. Development 108:229–238
Steinberg MS (1970) Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool 173:395–434
Suzuki A, Kuwabara K, Kuwabara Y (1975) Temporal relations between extension of archenteron roof and realization of neural induction during gastrulation of newt embryo. Dev Growth Differ 17:343–353
Suzuki AS, Matsuda J (1991) Neural-inducing activity and glycoprotein synthesis of newly mesodermalized ectoderm. Rouxs Arch Dev Biol (in press)
Suzuki AS, Nakatake H, Hidaka T (1984) Cell-to-cell contact in primary embryonic induction: effects of lectin on electrical coupling and neural induction. Differentiation 28:73–77
Suzuki AS, Yoshimura Y, Yano Y (1986a) Neural-inducing activity of newly mesodermalized ectoderm. Rouxs Arch Dev Biol 195:168–172
Suzuki AS, Ueno T, Matsusaka T (1986b) Alteration of cell adhesion system in amphibian ectoderm cells during primary embryonic induction: changes in reaggregation pattern of induced neuroectoderm cells and ultrastructural features of the reaggregate. Rouxs Arch Dev Biol 195:85–91
Tacke L, Grunz H (1988) Close juxtaposition between inducing chordamesoderm and reacting neuroectoderm is a prerequisite for neural induction inXenopus laevis. Cell Differ 24:33–44
Takata K, Yamazaki-Yamamoto K, Ozawa R (1981) Use of lectins as probes for analyzing embryonic induction. Rouxs Arch Dev Biol 190:92–96
Takeichi M (1988) The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102:639–655
Tiedemann H (1986) The molecular mechanism of neural induction: neural differentiation ofTriturus ectoderm exposed to Hepes buffer. Roux Arch Dev Biol 195:399–402
Tiedemann H, Born J (1978) Biological activity of vegetalizing and neuralizing inducing factors after binding to BAC-cellulose and CNBr-sepharose. Rouxs Arch Dev Biol 184:285–299
Townes PL, Holtfreter J (1955) Direct movements and selective adhesion of embryonic amphibian cells. J Exp Zool 128:53–120
Yamazaki-Yamamoto K, Ozawa R, Takata K, Kitoh J (1981) Cell surface changes of the presumptive ectoderm following neuralinducing treatment by concanavalin A. Rouxs Arch Dev Biol 190:313–319
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suzuki, A.S., Miyagawa, J. & Kuwana, T. Changes in the interior surface of newly mesodermalized ectoderm and its contact activity with competent ectoderm in the newtCynops (Amphibia). Roux's Arch Dev Biol 199, 413–422 (1991). https://doi.org/10.1007/BF01705852
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01705852