Summary
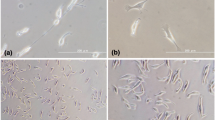
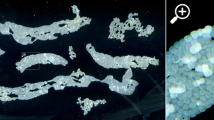
The hemocytes oftu-Sz ts melanotic tumor larvae ofDrosophila melanogaster encapsulate heterospecific and surface-modified homospecific tissue implants, but do not encapsulate unmodified homospecific implants (R. Rizki and Rizki 1980). In the present study we usedtu-Sz ts hosts to assay changes in larval fat body surfaces during development. Donor fat bodies from various ages of larvae were accepted (remained unencapsulated) intu-Sz ts hosts whereas fat bodies from donors with everted spiracles and all subsequent stages of development that were tested were rejected (encapsulated). Since the demarcation between acceptance and rejection by thetu-Sz ts blood cells did not coincide with the gross morphological changes that appear in the fat body during metamorphosis (dissolution of the basement membrane and dispersal of the freed fat body cells at pupation), we compared acceptable and nonacceptable fat body surfaces by three other methods. Fat body surface ultrastructure was examined, fat bodies were treated with fluorescein isothiocyanate-conjugated lectins, and fat body surfaces were reacted with a monoclonal antibody specific for basement membrane. These approaches did not uncover fat body surface changes associated with eversion of the anterior spiracles, suggesting that recognition of tissue surface heterogeneities by the insect hemocytes exceeds the resolving power of the other three methods. However, the monoclonal antibody fails to bind to the basement membrane ofD. virilis larvae, whose fat body is always rejected intu-Sz ts hosts. This supports our suggestion that the molecular architecture of the basement membrane may be important in eliciting the encapsulation response.
Similar content being viewed by others
References
Adams E (1978) Invertebrate collagens. Science 202:591–598
Ashhurst DE (1968) The connective tissue of insects. Ann Rev Entomology 13:45–74
Ashhurst DE, Bailey AJ (1980) Locust collagen: morphological and biochemical characterization. Eur J Biochem 103:75–83
Butterworth FM, Bodenstein D, King RC (1965) Adipose tissue ofDrosophila melanogaster. I. An experimental study of larval fat body. J Exp Zool 158:141–154
Callahan WP, Horner JA (1964) The use of vanadium as a stain for electron microscopy. J Cell Biol 20:350–356
Garen A, Kauvar L, Lepesant I (1977) Roles of ecdysone inDrosophila development. Proc Natl Acad Sci 74:5099–5103
Harper E, Seifter S, Scharrer B (1967) Electron microscopic and biochemical characterization of collagen in blattarian insects. J Cell Biol 33:385–393
Hodgetts RB, Sage B, O'Connor JD (1977) Ecdysone titers during postembryonic development ofDrosophila melanogaster. Dev Biol 60:310–317
Köhler G, Milstein C (1976) Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol 6:511–519
Peters BP, Goldstein IJ (1979) The use of fluorescein-conjugatedBandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of α-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res 120:321–334
Poulson DF (1950) Histogenesis, organogenesis, and differentiation in the embryo ofDrosophila melanogaster Meigen. In: Demerec (ed) Biology ofDrosophila. John Wiley and Sons, New York, pp 168–274
Rizki TM (1961) Intracellular localization of kynurenine in the fat body ofDrosophila. J Biophys Biochem Cytol 9:567–572
Rizki TM (1978) Fat Body. In: Ashburner M, Wright TRF (eds) The genetics and biology ofDrosophila, vol 2b. Academic Press, London, pp 561–601
Rizki RM, Rizki TM (1979) Cell interactions in the differentiation of a melanotic tumor inDrosophila. Differentiation 12:167–178
Rizki TM, Rizki RM (1980) Developmental analysis of a temperature-sensitive melanotic tumor mutant inDrosophila melanogaster. Wilhelm Roux's Arch 189:197–206
Rizki RM, Rizki TM (1980) Hemocyte responses to implanted tissues inDrosophila melanogaster larvae. Wilhelm Roux's Arch 189:207–213
Rizki RM, Rizki TM, Andrews CA (1975)Drosophila cell fusion induced by wheat germ agglutinin. J Cell Sci 18:113–121
Rizki RM, Rizki TM, Andrews CA (1977) Modification ofDrosophila cell surfaces by Concanavalin A. Cell Tissue Res 185:183–190
Roberts DB, Wolfe J, Akam ME (1977) The developmental profiles of two major haemolymph proteins fromDrosophila melanogaster. J Insect Physiol 23:871–898
Seecof RL (1971) Phosphate-buffered saline forDrosophila. Drosophila Information Service 46:113
Spragg JH, Bebbington CR, Roberts DB (1982) Monoclonal antibodies recognizing cell surface antigens inDrosophila melanogaster. Dev Biol 89:339–352
Thomasson WA, Mitchell HK (1972) Hormonal control of protein granule accumulation in fat bodies ofDrosophila melanogaster larvae. J Insect Physiol 18:1885–1899
Thomson JA (1975) Major patterns of gene activity during development in holometabolous insects. Adv Insect Physiol 11:321–398
Whitten JM (1962) Breakdown and formation of connective tissue in the pupal stage of an insect. Quart J Micro Sci 103:359–367
Wigglesworth VB (1949) The utilization of reserve substances inDrosophila during flight. J Exp Biol 26:150–163
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rizki, R.M., Rizki, T.M., Bebbington, C.R. et al. Drosophila larval fat body surfaces. Wilhelm Roux' Archiv 192, 1–7 (1983). https://doi.org/10.1007/BF00848762
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00848762