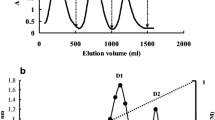
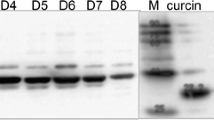
Abstract
Petrocoptis glaucifolia, a paleoendemic member of the Caryophyllaceae from the North of Spain, was found to contain at least five proteins that inhibit protein synthesis in a rabbit reticulocyte lysate. One of them, for which the name petroglaucin is proposed, was purified to apparent electrophoretic homogeneity by chromatography through S-Sepharose Fast Flow, Sephadex G-75 and CM-Sepharose Fast Flow. The apparent Mr of the preparation was 27500. This protein does not contain appreciable glycan chains and displays 45.8% of NH2-terminal amino-acid sequence homology with some ribosome-inactivating proteins from Saponaria officinalis, another member of the Caryophyllaceae. Petroglaucin shows the following functional properties: (i) it strongly inhibits the rabbit-reticulocyte-lysate system and Vicia sativa cell-free extracts, both coded by endogenous messengers, and also inhibits poly(U)-directed polyphenylalanine synthesis by Vicia sativa cell-free extracts and purified rat-liver ribosomes; (ii) it shows much less inhibitory capacity in wheat-germ, Cucumis sativus and rat-liver cell-free systems coded by endogenous messengers; (iii) the inhibitory effects on purified rat-liver ribosomes were irreversible; (vi) it promotes the release of adenine from purified rat-liver ribosomes. The total activity of this translational inhibitor has been found to increase up to 11-fold during its purification, indicating that some regulatory factor that normally blocks the translational inhibitory activity of the ribosome-inactivating protein in crude extracts of the plant is removed during purification.
Similar content being viewed by others
Abbreviations
- DFA:
-
dimethylforrnamide
- PAGE:
-
polyacrylamide gel electrophoresis
- poly(U):
-
polyuridylic acid
- RIP:
-
ribosome inactivating protein
- SDS:
-
sodium dodecyl sulfate
References
Barber, D., Limas, G.G., Gavilanes, J.G., Méndez, E. (1988) Isolation and characterization of thirteen new salt-soluble proteins from barley by reverse-phase high-performance liquid chromatography. Planta 176, 221–229
Barbieri, L., Stirpe, F. (1982) Ribosome-inactivating proteins from plants: properties and possible uses. Cancer Surveys 1, 489–520
Barbieri, L., Stoppa, C., Bolognesi, A. (1987) Large scale Chromatographic purification of ribosome-inactivating proteins. J. Chromatogr. 408, 235–243
Barbieri, L. Bolognesi, A., Cenini, P., Falasca, A., Minghetti, A., Garofano, L., Guicciardi, A., Lappi, D., Miller, S., Stirpe, F. (1989). Ribosome-inactivating proteins from plant cells in culture. Biochem. J. 257, 801–807
Batelli, M.G., Lorenzoni, E., Stirpe, F., Cella, R., Parisi, B. (1984) Differential effect of ribosome-inactivating proteins on plant ribosome activity and plant cell growth. J. Exp. Bot. 35, 882–889
Bjorn, M.I., Larrick, J., Piatak, M., Wilson, K.J. (1984) Characterization of translational inhibitors from Phytolacca americana. Amino-terminal sequence determination and antibody-inhibitor conjugates. Biochim. Biophys. Acta 790, 154–163
Bolognesi, A., Barbieri, L., Carnicelli, D., Abbondanza, A., Cenini, P., Falasca, A.I., Dinota, A., Stirpe, F. (1989) Purification and properties of a new ribosome-inactivating protein with RNA N-glycosidase activity suitable for immunotoxin preparation from the seeds of Momordica conchinchinensis. Biochim. Biophys. Acta 993, 287–292
Bradford, M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Brigotti, M., Rambelli, F., Zamboni, M., Montanaro, L., Sperti, S. (1989) Effect of α-sarcin and ribosome-inactivating proteins on the interaction of elongation factors with ribosomes. Biochem. J. 257, 723–727
Cenini, P., Batelli, M.G., Bolognesi, A., Stirpe, F., Villenez, C.L. (1987) Effect of ribosome-inactivating proteins on ribosomes from Tetrahymena pyriformis and Acanthamoeba castellani. Biochem. Biophys. Res. Commun. 148, 521–527
Cenini, P., Bolognesi, A., Stirpe, F. (1988) Ribosome-inactivating proteins from plants inhibit ribosome activity of Trypanosoma and Leishmania. J. Protozool. 35, 384–387
Endo, Y., Tsurugi, K. (1987) RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 262, 8128–8130
Ferreras, J.M., Iglesias, R., Merino, M.J., Girbés, T. (1989a) Presence of translational inhibitory activity in partially purified extracts from two Petrocoptis species. Cell. Mol. Biol. 35, 89–95
Ferreras, J.M., Merino, M.J., Iglesias, R., Muñoz, R., Girbés, T. (1989b) Isolation of a ribosome-inactivating type-1 protein of Cucumis melo. Biochem. Int. 19, 201–207
Frankel, A.E., Houston, L.L., Issell, B.F., Fatham, G. (1986) Prospects for immunotoxin therapy in cancer. Annu. Rev. Med. 37, 125–142
Gasperi-Campani, A., Barbieri, L., Batelli, M.J., Stirpe, F. (1985) On the distribution of the ribosome-inactivating proteins amongst plants. J. Nat. Prod. 48, 446–454
Gasperi-Campani, A., Barbieri, L., Morelli, P., Stirpe, F. (1980) Seed extracts inhibiting protein synthesis in vitro. Biochem. J. 186, 439–441
Girbés, T., Cabrer, B., Modolell, J. (1979) Preparation and assay of purified Escherichia coli polysomes devoid of free ribosomal subunits and endogenous GTPase activities. Methods Enzymol. 59, 353–362
Girbés, T., Ferreras, J.M., Muñoz, R., Alonso, P. (1989) Effect of acute ethanol administration and nutritional status on secretory protein synthesis in isolated rat liver cells. Toxic. In vitro 3, 7–12
Girbés, T., Susin, A., Ayuso, M.S., Parrilla, R. (1983) Acute effects of ethanol in the control of protein synthesis in isolated rat liver cells. Arch. Biochem. Biophys. 226, 37–49
Harley, S., Beevers, H. (1982) Ricin inhibition of in vitro protein synthesis by plant ribosomes. Proc. Natl. Acad. Sci. USA 79, 5935–5938
Hopp, T., Woods, K.R. (1981) Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78, 3824–3828
Jimenez, A., Vázquez, D. (1985) Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu. Rev. Microbiol. 39, 649–672
Kishida, K., Masuho, Y., Hara, T. (1983) Protein-synthesis inhibitory protein from seeds of Luffa cylindrica Roehm. FEBS Letters 153, 209–212
Koppel, G.A. (1990) Recent advances with monoclonal antibody drug targeting for the treatment of human cancer. Bioconj. Chem. 1, 13–23
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Lappi, D.A., Esch, F.S., Barbieri, L., Stirpe, F., Soria, M. (1985) Characterization of a Saponaria officinalis seed ribosome-inactivating protein: immunoreactivity and sequence homologies. Biochem. Biophys. Res. Commun. 129, 934–942
Limas, G.G., Salinas, M., Monco, L, Fischer, S., Wittman-Liebold, B., Mendez, E. (1990) Purification and characterization of new rice NaCl-soluble proteins: identification of four protein-synthesis inhibitors and two immunoglobulin-binding proteins. Planta 181, 1–9
Lord, J.M. (1987) The use of cytotoxic plant lectins in cancer therapy. Plant Physiol. 85, 1–3
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with Folin-phenol reagent. J. Biol. Chem. 193, 265–275
Martin, A., Perez, J., Ayuso, M.S., Parrilla, R. (1979) On the mechanism of the glucagon-induced inhibition of hepatic protein synthesis. Arch. Biochem. Biophys. 195, 223–234
McCann, W.P., Hall, L.M., Siler, W., Barton, N., Whitley, R.J. (1985) High-pressure liquid Chromatographic methods for determining arabinosyladenine-5′-monophosphate, arabinosyladenine, and arabinosylhipoxantine in plasma and urine. Antimicrob. Agents Chemother. 28, 265–273
McGrath, M.S., Hwang, K.M., Caldwell, S.E., Gaston, L, Luk, K.C., Wu, P., Ng, V.L., Crowe, S., Daniels, J., Marsh, J., Deinhart, T., Lekas, P.V., Vennari, J.C., Yeung, H.W., Lifson, J.D. (1989) GLQ 223: An inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA 86, 2844–2848
Merino, M.J., Ferreras, J.M., Muñoz, R., Iglesias, R., Girbés, T. (1990) Plant species containing inhibitors of eukaryotic protein synthesis. J. Exp. Bot. 41, 67–70
Montanaro, L., Sperti, S., Zamboni, M., Denaro, M., Testoni, G., Gasperi-Campani, A., Stirpe, F. (1978) Effect of modeccin on the steps of peptide-chain elongation. Biochem. J. 176, 371–379
Montecucchi, P.C., Lazzarini, A.M., Barbieri, L., Stirpe, F., Soria, M., Lapi, D. (1989) N-terminal sequence of some ribosome-inactivating proteins, Int. J. Peptide Protein Res. 33, 263–267
Muñoz, R., Ferreras, J.M., Iglesias, R., Merino, M.J., Girbés, T. (1990a) Adaptation of in vitro rat brain protein synthesis to long-term ingestion of n-butanol. Brain Res. 517, 330–332
Muñoz, R., Ferreras, J.M., Iglesias, R., Merino, M.J., Girbés, T. (1990b) Messenger-dependent action of guanylylimidodiphosphate and translation inhibitors on rat brain polypeptide synthesis. Cell. Mol. Biol. 36, 329–335
Pelham, H.D.B., Jackson, R.J. (1976) An efficient mRNA-dependent translation system from reticulocyte lysates. Eur. J. Biochem. 67, 247–256
Roberts, W.K., Selitrennikoff, C.P. (1986) Plant proteins that inactivate foreign ribosomes. Biosci. Rep. 6, 19–29
Singh, V., Sairam, M.R., Bhargavi, G.N., Akhras, R.G. (1989) Hormonotoxins. Preparation and characterization of ovine luteinizing hormone-gelonin conjugate. J. Biol. Chem. 264, 3089–3095
Stahelin, T., Falvey, A.K. (1971) Isolation of mammalian ribosomal subunits active in polypeptide synthesis. Methods Enzymol. 20, 433–446
Stirpe, F., Barbieri, L. (1986) Ribosome-inactivating proteins up to date. FEES Lett. 195, 1–8
Stirpe, F., Hughes, C. (1989) Specificity of ribosome-inactivating proteins with RNA N-glycosidase activity. Biochem. J. 262, 1001–1002
Stirpe, F., Olsness, S., Pihl, A. (1980) Gelonin, a new inhibitor of protein synthesis, non-toxic to intact cells. Isolation, characterization and preparation of cytotoxic conjugates with concanavalin A. J. Biol. Chem. 225, 6947–6953
Stirpe, F., Williams, D.G., Onyon, L.J., Legg, R.F., Stevens, W.A. (1981) Dianthins, ribosome-damaging proteins with antiviral properties from Dianthus Caryophyllus L. (carnation). Biochem. J. 195, 399–405
Stirpe, F., Barbieri, L., Batelli, M.G., Falasca, A., Lorenzoni, E., Stevens, W.A. (1986) Bryodin a ribosome-inactivating protein from the roots of Bryonia dioica L. (White bryony). Biochem. J. 240, 659–665
Stirpe, F., Bailey, S., Miller, S.P., Bodley, J.W. (1988) Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 16, 1349–1357
Till, M., Ghetie, V., Gregory, T., Patzer, E., Porter, J.P., Uhr, J.W., Capon, D.J., Vitetta, E.S. (1988) HIV-infected cells are killed by rCD4-ricin A chain. Science 242, 1166–1168
Vitetta, E.S., Uhr, J.W. (1985) Immunotoxins. Annu. Rev. Immunol. 3, 197–212
Zamboni, M.C., Brigotti, M., Rambelli, F., Montanaro, L., Sperti, S. (1989) High-pressure-liquid-chromatographic and fluorimetric methods for the determination of adenine release from ribosomes by ricin and gelonin. Biochem. J. 259, 639–643
Author information
Authors and Affiliations
Additional information
The work in Valladolid was supported by grants from CICYT (BIO 88-0705), Junta de Castilla y León and Iberduero S.A. The work in Bologna was supported by grants from Ministerio della Pubblice Istruzione, Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale dell Ricerche, within the Progetto finalizzato Biotenologia e biostrumentazione. F.J. Arias and M.A. Rojo hold fellowships from Iberduero S.A. J.M. Ferreras and R. Iglesias hold postdoctoral fellowships from Ministerio de Educación y Ciencia. We thank Professor R. Parilla (Instituto Gregorio Marañon, Madrid) for his critical reading of the manuscript. The supervision of the English version of the manuscript by N. Skinner is greatly appreciated.
Rights and permissions
About this article
Cite this article
Arias, F.J., Rojo, M.A., Ferreras, J.M. et al. Isolation and partial characterization of a new ribosome-inactivating protein from Petrocoptis glaucifolia (Lag.) Boiss. Planta 186, 532–540 (1992). https://doi.org/10.1007/BF00198033
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198033