Abstract
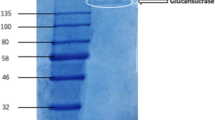
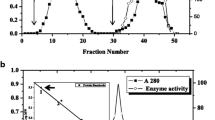
The plasma-membrane-localized 1,3-β-glucan synthase (EC 2.4.1.34) from suspension cultures of Glycine max (L.) Merr. was greatly enriched by a three-step purification procedure. Starting with a microsomal preparation, a six- to eightfold enrichment of the enzyme was achieved by isolating plasma-membrane vesicles in a polyethyleneglycol/dextran two-phase system. The enzyme was solubilized with the nonionic detergent digitonin and further purified 12-fold by successive centrifugations on two linear sucrose density gradients. The most purified enzyme preparation showed enrichment in a 31-kilodalton (kDa) polypeptide and was used to raise polyspecific antibodies which precipitated 1,3-β-glucan synthase activity. These antibodies were purified by affinity chromatography against immobilized membrane protein fractions of lower molecular weight which were devoid of 1,3-β-glucan synthase activity. The purified antibodies specifically labelled a single polypeptide of 31 kDa in the 1,3-β-glucan-synthase-containing heavy fractions of the first sucrose gradient indicating that this polypeptide represents part of the active enzyme complex.
Similar content being viewed by others
Abbreviations
- CHAPS:
-
3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonic acid
- SDS-PAGE:
-
sodium dodecyl sulfatepolyacrylamide gel electrophoresis
References
Bethell, G.S., Ayers, J.S., Hancock, W.S. (1979) A novel method of activation of cross-linked agaroses with 1,1′-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J. Biol. Chem. 254, 2572–2574
Bradford, M.M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Delmer, D.P. (1987) Cellulose biosynthesis. Annu. Rev. Plant Physiol. 38, 259–290
Delmer, D.P., Solomon, M. (1989) Identification of polypeptides involved in plant β-glucan synthesis. In: Abstr., 5th Cell Wall Meeting, Edinburgh, Abstr. 36
Fink, J., Jeblick, W., Blaschek, W., Kauss, H. (1987) Calcium ions and polyamines activated the plasma membrane-located 1,3-β-glucan synthase. Planta 171, 130–135
Giddings, T.H., Brower, D.L., Staehelin, L.A. (1980) Visualization of particle complexes in the plasma membrane of Micrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary walls. J. Cell. Biol. 84, 327–339
Gordon, R., McLachlan, G. (1989) Incorporation of UDP-[14C]glucose into xyloglucan by pea membranes. Plant Physiol. 91, 373–378
Jacob, S.R., Northcote, D.H. (1985) In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J. Cell Sci. Suppl. 2, 1–11
Kauss, H. (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu. Rev. Plant Physiol. 38, 47–72
Kauss, H., Jeblick, W. (1987) Solubilization, affinity chromatography and Ca2+ /polyamine activation of the plasma membraneactivated 1,3-β-D-glucan synthase. Plant Sci. 48, 63–69
Kauss, H., Köhle, H., Jeblick, W. (1983) Proteolytic activation and stimulation by Ca2+ of the glucan synthase from soybean cells. FEBS Lett. 158, 84–88
Kauss, H., Waldmann, T., Quader, H. (1990) Ca2+ as a signal in the induction of callose synthesis. In: Signal perception and transduction in higher plants, Ranjeva, R., Boudet, A., eds. Springer, Berlin Heidelberg New York, in press
Kjellbom, P., Larsson, C. (1984) Preparation and polypeptide composition of chlorophyll-free plasma membranes from leaves of light-grown spinach and barley. Physiol. Plant 62, 501–509
Köhle, H., Jeblick, W., Poten, F., Blaschek, W., Kauss, H. (1985) Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol. 77, 544–551
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Lawson, S.G., Mason, T.L., Sabin, R.D., Sloan, M.E., Drake, R.R., Haley, B.E., Wasserman, B.P. (1989) UDP-glucose: (1,3)-β-glucan synthase from Daucus carota L. Plant Physiol. 90, 101–108
Ogata, K., Arakawa, M., Kasahara, T., Shiori-Nakano, K., Hiraoka, K. (1983) Detection of toxoplasma membrane antigens transferred from SDS-polyacrylamide gel to nictrocellulose with monoclonal antibody and avidin-biotin, peroxidase antiperoxidase and immunoperoxidase methods. J. Immunol. Methods 65, 75–82
Read, S.M., Delmer, D.P. (1987) Inhibition of mung bean UDP-glucose: (1->3)-β-glucan synthase by UDP-pyridoxal. Evidence for an active-site amino group. Plant Physiol. 85, 1008–1015
Robinson, D.G., Quader, H. (1981) Structure, synthesis and orientation of microfibrils. IX. A freeze-fracture investigation of the Oocystic plasma membrane after inhibitor treatment. Eur. J. Cell Biol. 25, 278–288
Shimazaki, Y., Pratt, L.H. (1985) Immunochemical detection with rabbit polyclonal and mouse monoclonal antibodies of different pools of phytochrome from etiolated and green Avena shoots. Planta 164, 333–334
Waldmann, T., Jeblick, W., Kauss, H. (1988) Induced net Ca2+ uptake and callose biosynthesis in suspension-cultured plant cells. Planta 173, 88–95
Wasserman, B.P., McCarthy, K.J. (1986) Regulation of plasma membrane β-glucan synthase from red beet root by phospholipids. Plant Physiol. 82, 396–400
Wasserman, B.P., Frost, D.J., Wu, A., Reed, S.M. (1989) Identification of the UDPG-binding polypeptide of (1,3)-β-glucan synthase of higher plants by photoaffinity labeling with 5-Azido-UDPG. In: Abstr., 5th Cell Wall Meeting, Edinburgh, Abstr. 35
Wessel, D., Flügge, U.I. (1984) A method for the quantitative recovery of protein in dilute solutions in the presence of detergents and lipids. Anal. Biochem. 138, 141–143
Author information
Authors and Affiliations
Additional information
Financial support of the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie is acknowledged. We also thank T.J. Buckhout for many suggestions and for correcting the English manuscript.
Rights and permissions
About this article
Cite this article
Fink, J., Jeblick, W. & Kauss, H. Partial purification and immunological characterization of 1,3-β-glucan synthase from suspension cells of Glycine max . Planta 181, 343–348 (1990). https://doi.org/10.1007/BF00195886
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195886