Abstract
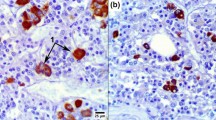
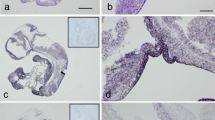
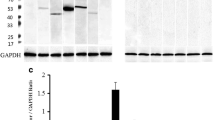
An immunohistochemical study was undertaken, in an attempt to identify the acidic glycoprotein(s) present in colloid and in parenchymal cells in human fetal pituitary gland. As the colloid has been proposed to represent disintegrating cells, a series of antibodies against plasma glycoproteins and plasma proteins was applied; their presence intracellularly would generally be an indicator of plasma membrane leakage in dying parenchymal cells. In tissue sections from 9- to 20-week-old fetuses, the colloid showed prominent staining with an antibody to human fetuin/α2 HS glycoprotein. Anti-α2-HS glycoprotein labelled parenchymal cells in pars anterior and intermedia. Apart from a minor immunoreactivity for α1 β glycoprotein, no other plasma glycoprotein was seen in colloid or parenchymal cells. An antibody against bovine fetuin showed staining of the colloid and of some parenchymal cells in pars distalis and intermedia; the plasma and stroma of the pituitary gland were unstained. In contrast, the anti-human plasma protein antibodies all stained the stroma. The presence of α2 HS glycoprotein in parenchymal cells and absence of other plasma glycoproteins imply integrity of the parenchymal cell plasma membrane. Thus, α2 HS glycoprotein is either synthesized locally or taken up specifically in the parenchymal cells, which are proposed to participate in the formation of colloid. It is suggested that α2 HS glycoprotein is part of a homeostatic system, which controls remodelling and physiological cell death during development.
Similar content being viewed by others
References
Allaerts W, Carmeliet P, Denef C (1990) Review: New perspectives in the function of pituitary folliculo-stellate cells. Mol Cell Endocrinol 71:73–81
Andersen H, Bülow FA von, Møllgård K (1970a) The histochemical and ultrastructural basis of the cellular function of the human foetal adenohypophysis. Prog Histochem Cytochem 1:1–32
Andersen H, Møllgård K, Bülow FA von (1970b) On the specificity of staining by Alcian blue in the study of human, foetal adenohypophysis. Histochemie 22:362–375
Andersen H, Bülow FA von, Møllgård K (1971) The early development of the pars distalis of the human foetal pituitary gland. Z Anat Entwickl-Gesch 135:117–138
Asa SL, Kovacs K, Laszlo FA, Domokos I, Ezrin C (1986) Human fetal adenohypophysis. Histologic and immunocytochemical analysis. Neuroendocrinology 43:308–316
Asa SL, Kovacs K, Horvath E, Losinski NE, Laszlo FA, Domokos I, Halliday WC (1988) Human fetal adenohypophysis. Electron microscopic and ultrastructural immunocytochemical analysis. Neuroendocrinology 48:423–431
Ashton BA, Smith R (1980) Plasma α2 HS glycoprotein concentration in Page's disease of bone: its possible significance. Clin Sci 58:435–438
Bergland RM, Torack RM (1969) An ultrastructural study of follicular cells in the human anterior pituitary. Am J Pathol 57:273–297
Brown WM, Dziegielewska KM, Møllgård K, Saunders NR (1992) Fetuin: An old friend revisited. Bioessays 15:1–7
Christie DL, Dziegielewska KM, Hill RM, Saunders NR (1987) Fetuin: the bovine homologue of α2 HS glycoprotein. FEBS Lett 214:45–49
Dickson IR, Poole AR, Veis A (1975) Localization of plasma α2 HS glycoprotein in mineralizing human bone. Nature 256:430–432
Dziegielewska KM, Møllgård K, Reynolds ML, Saunders NR (1987) A fetuin-related glycoprotein (α2 HS) in human embryonic and fetal development. Cell Tissue Res 248:33–41
Dziegielewska KM, Brown WM, Casey S-J, Christie DL, Foreman RC, Hill RM, Saunders NR (1990) The complete cDNA and amino acid sequence of bovine fetuin. Its homology with α2 HS glycoprotein and relation to other members of the cystatin superfamily. J Biol Chem 265:4354–4357
Dziegielewska KM, Habgood MD, Møllgård K, Stagaard M, Saunders NR (1991) Species specific transfer of plasma albumin from blood into different cerebrospinal fluid compartments in the immature fetal sheep. J Physiol 439:215–237
Farquhar MG (1957) “Corticotrophs” of the rat adenohypophysis as revealed by electron microscopy. Anat Rec 127:291
Harboe NHG, Ingild A (1983) Immunization, isolation of immunoglobulins and antibody titre determination. Scand J Immunol 17, Suppl 10:245–351
Horvath E, Kovacs K, Penz G, Ezrin C (1974) Origin, possible function and fate of “follicular cells” in the anterior lobe of the human pituitary. Am J Pathol 77:199–212
Ishikawa Y, Wu LNY, Valhmu WB, Wuthier RE (1991) Fetuin and alpha-2HS glycoprotein induce alkaline phosphatase in epiphyseal growth plate chondrocytes. J Cell Physiol 149:222–234
Kellermann J, Haupt A, Auerswald E-A, Müller-Esterl W (1989) The arrangement of disulfide loops in human α2 HS glycoprotein: similarity to the disulfide bridge structures of cystatins and kininogens. J Biol Chem 264:14121–14128
Knox P, Griffiths S, Whately JG (1979) Fetal calf serum and the adhesion of cells in culture. Biochem Soc Trans 7:1011–1012
Lee C-C, Bowman BH, Yang F (1987) Human α2-HS-glycoprotein: the A and B chains with a connecting sequence are encoded by a single mRNA transcript. Proc Natl Acad Sci USA 84:4403–4407
Lewis JG, André CM (1978) A serum DNA-binding protein absent in malignant disease. FEBS Lett 92:211–213
Lewis JG, André CM (1981) Enhancement of human monocyte phagocytic function by α2 HS glycoprotein. Immunology 42:481–487
Møllgård K, Balslev Y (1989) The subcellular distribution of transferrin in rat choroid plexus studied with immunogold labelling of ultracryosections. Histochem J 21:441–448
Oss CJ van, Gillman CF, Bronson PM, Border JR (1974) Opsonic properties of human serum α2 HS glycoprotein. Immunol Commun 3:329–335
Reynolds ML, Møllgård K, Saunders NR (1983) The distribution of plasma proteins during early embryonic development in the sheep. Anat Embryol 168:227–240
Rizzino A, Sherman MI (1979) Development and differentiation of mouse blastocysts in serum free medium. Exp Cell Res 121:221–233
Selye H (1943) Experiments concerning the mechanism of pituitary colloid secretion. Anat Rec 86:109–119
Triffit JT, Gebauer U, Ashton BA, Owenn ME, Reynolds JJ (1976) Origin of plasma α2 HS glycoprotein and its accumulation in bone. Nature 41:226–227
Yang F, Chen Z-L, Bergeron JM, Cupples RL, Friederichs WE (1992) Human α2-HS-glycoprotein/bovine fetuin homologue in mice: identification and developmental regulation of the gene. Biochem Biophys Acta 1130:149–156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
von Bülow, F.A., Janas, M.S., Terkelsen, O.B.F. et al. Human fetuin/α2 HS glycoprotein in colloid and parenchymal cells in human fetal pituitary gland. Histochemistry 99, 13–22 (1993). https://doi.org/10.1007/BF00268015
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00268015