Abstract
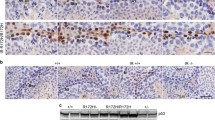
The testis is a tissue of high proliferative activity. In this organ, sperm cells (spermatozoa) are produced from stem cells (spermatogonia) by two consecutive steps of cell multiplication and spermatid cytodifferentiation. Mitotic proliferation of spermatogonia generates primary spermatocytes which enter meiosis, leading to the generation of spermatids. The number of cells entering meiosis is held constant, since outnumbering spermatogonia or premeiotic spermatocytes are eliminated by apoptosis (programmed cell death). During apoptosis, the nuclear chromatin is internucleosomally degraded by the activity of a Ca2+, Mg2+-dependent endonuclease. Recent data indicate that deoxyribonuclease I (DNase I) is identical to the apoptotic endonuclease responsible for the internucleosomal DNA degradation. Previous results using primers specific for rat parotid DNase I in a polymerase chain reaction have demonstrated the presence of DNase I-specific gene transcripts in rat testis. We have therefore analysed the presence of DNase I in rat testis by immunohistochemistry and biochemical procedures. The presence of DNase I-like endonucleolytic activity was verified enzymatically. DNase I immunoreactivity was detected in the nuclei of a few spermatogonia and premeiotic spermatocytes, but within the acrosomic vesicle of all spermatids and spermatozoa. In situ hybridisation revealed the accumulation of DNase I-specific gene transcripts in a small number of spermatogonia and/or premeiotic spermatocytes, but in a large number of spermatids. The occurrence of apoptotic DNA fragmentation was investigated by in situ end-labelling (ISEL) of free 3′-OH DNA ends and gave positive nuclear staining of only very few spermatogonia. No positive ISEL staining was observed in maturing spermatids and/or spermatozoa. These data support the notion that, within the seminiferous epithelium, the number of primary spermatocytes entering meiosis is controlled by apoptosis. In addition, they demonstrated that mature sperm cells are equipped with an endonuclease that might be used for DNA degradation during their elimination at later stages of their life span. The expression and distribution of the tumour suppressor gene product, p53, was analysed by immunostaining. Strong p53 immunoreactivity was observed in the nuclei of a number of spermatogonia, of some premeiotic spermatocytes and probably in all spermatids. Thus, p53 expression appeared to parallel that of DNase I. In contrast, p53 immunoreactivity was absent in mature spermatozoa present in the lumen of the testicular tubules or the ductus epididymidis. It is therefore proposed that at later stages of spermatid maturation— most probably before their release as mature spermatozoa —the p53 gene product was either degraded or retained in residual bodies, since p53 immunoreactivity was found to be concentrated within these organelles.
Similar content being viewed by others
References
Bartke A (1995) Editorial: apoptosis of male germ cells: a generalized or a cell type-specific phenomenon? Endocrinology 136:3–4
Clarke PGH (1990) Developmental cell dealth: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN (1992) Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J 286:331–334
Duvall E, Wyllie AH, Morris RG (1985) Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56:351–358
Ellis RE, Yuan J, Horvitz HR (1991) Mechanisms and functions of cell death. Annu Rev Cell Biol 7:663–698
Gavrieli Y, Sherman Y, Ben-Sasson AB (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Hilscher B, Hilscher W, Bülthoff-Ohnholz B, Krämer U, Birke A, Pelzer H, Gauss G (1974) Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res 154:443–470
Horstmann E, Richter R, Roosen-Runge E (1966) Zur Elektronenmikroskopie der Kerneinschlüsse im menschlichen Nebenhodenepithel. Z Zellforsch 69:69–79
Huckins C (1978) The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplifield classification of the germinal epithelium. Anat Rec 190:905–926
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kreuder V, Dieckhoff J, Sittig M, Mannherz HG (1984) Isolation, characterization and crystallisation of deoxyribonuclease I from bovine and rat parotid gland and its interaction with rabbit skeletal muscle actin. Eur J Biochem 139:389–400
Lane DP (1992) p53, guardian of the genome. Nature 358:15–16
Leithäuser F, Dhein J, Mechtersheimer G, Koretz K, Brüderlein S, Henne C, Schmidt A, Debathin K-M, Krammer PH, Möller P (1993) Constitutive and included expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest 69:415–429
Lutter LC (1978) Characterization of DNase I cleavage sites in the nucleosome. Cold Spring Harbor Symp Quant Biol 42:137–147
Mannherz HG, Goody RS, Konrad M, Nowak E (1980) The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochim 104:367–379
Mortimer D, Curtis EF, Miller RG (1987) Specific labelling by peanut agglutinin of the outer acrosomal membrane of the human spermatozoa. J Reprod Fertil 81:127–135
Nadano D, Yasuda T, Kishi K (1993) Measurement of deoxyribonuclease I activity in human tissues and body fluids by a single radial enzyme-diffusion method. Clin Chem 39:448–452
Oberhammer F, Wilson FW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M (1993) Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12:3679–3684
Peitsch MC, Hesterkamp T, Polzar B, Mannherz HG, Tschopp J (1992) Functional characterisation of serum DNase I in MRL-ipr/Ipr mice. Biochem Biophys Res Commun 186:739–745
Peitsch MC, Polzar B, Stephan H, Crompton T, MacDonald HR, Mannherz HG, Tschopp J (1993) Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J 12:371–377
Peitsch MC, Mannherz HG, Tschopp J (1994a) The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol 4:37–41
Peitsch MC, Polzar B, Tschopp J, Mannherz HG (1994b) About the involvement of deoxyribonuclease I in apoptosis. Cell Death Differ 1:1–6
Polzar B, Mannherz HG (1990) Nucleotide sequence of a full length cDNA clone encoding the deoxyribonuclease I from rat parotid gland. Nucleic Acids Res 18:7151
Polzar B, Rösch A, Mannherz HG (1989) A simple procedure to produce monospecific polyclonal antibodies of high affinity against actin from muscular sources. Eur J Cell Biol 50:220–229
Polzar B, Peitsch MC, Loos R, Tschopp J, Mannherz HG (1993) Overexpression of deoxyribonuclease I (DNase I) transfected into COS-cells: its distribution during apoptotic cell death. Eur J Cell Biol 62:397–405
Polzar B, Zanotti S, Stephan H, Rauch F, Peitsch MC, Irmler M, Tschopp J, Mannherz HG (1994) Distribution of deoxyribonuclease I in rat tissues and its correlation to cellular turnover and apoptosis. Eur J Cell Biol 64:200–210
Regaud CP (1900) Degenerescence des cellules seminalez chez mammiferes en l'absence de tout état pathologique. O R Searces Soc Biol Fil 52:268–270
Roldan ERS, Harrison RAP (1990) Molecular mechanisms leading to exocytosis during the sperm acrosome reaction. In: Bavister BD, Cummins J, Roldan ERS (eds) Fertilization in mammals. Serono Symposia, Norwell, USA
Roosen-Runge EC (1955) Untersuchungen über die Degeneration samenbildender Zellen in der normalen Spermatogenese der Ratte. Z Zellforsch 41:221–235
Roosen-Runge EC (1964) The degeneration of pre-spermatogonial germ cells in the rat after birth. Anat Rec 148:328
Rosenthal AL, Lacks SA (1977) Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem 80:76–90
Russel LD, Ettlin RA, Sinha-Hikim AP, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache-River Press, Clearwater, USA
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schägger H, Jagow G von (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231
Schägger H, Cramer WA, Jagow G von (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217:220–230
Schwartz D, Goldfinger N, Rotter V (1993) Expression of p53 protein in spermatogenesis in confined to the tetraploid pachytene primary spermatocytes. Oncogene 8:1487–1494
Stöhr P (1886) Lehrbuch der Histologic und mikroskopischen Anatomie des Menschen. Gustav Fischer, Jena
Tesarik J (1990) Biology of human fertilization: the general and the special. In: Evers JHL, Heipemann MJ (eds) From ovulation to implantation. Elsevier, Amsterdam
Wartenberg H (1976) Comparative cytomorphologic aspects of the male germ cells, especially of the “gonia”. Andrologia 8:117–130
Wijsman JH, Jonker RR, Keijzer R, Velde JH van de, Conrelisse CJ, Dierendonck JH van (1993) A new method to detect apoptosis in paraffin sections: in situ end labeling of fragmented DNA. J Histochem Cytochem 41:7–12
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Zanotti S, Polzar B, Stephan H, Doll U, Niessing J, Mannherz HG (1995) Localization of deoxyribonuclease I gene transcripts and protein in rat tissues and its correlation with apoptotic cell elimination. Histochemistry 103:369–377
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stephan, H., Polzar, B., Rauch, F. et al. Distribution of deoxyribonuclease I (DNase I) and p53 in rat testis and their correlation with apoptosis. Histochem Cell Biol 106, 383–393 (1996). https://doi.org/10.1007/BF02473297
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02473297