Abstract
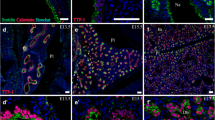
Tissue distribution and developmental expression of fetuin were studied in the sheep fetus from embryonic day (E) 30 to adult (gestational period is 150 days). The presence of fetuin was demonstrated immunocytochemically using anti-fetuin antibodies; in situ hybridisation using short anti-sense oligonucleotide probes labelled with digoxigenin was used to study the ability of the developing tissue to synthesise fetuin, and reverse transcription-polymerase chain reaction (RT-PCR) was used to estimate the level of fetuin mRNA in selected tissues. Tissue distribution of fetuin was widespread in the younger fetuses (E30 to E40). The most prominent presence due to in situ synthesis was demonstrated in the liver, central nervous system (CNS) including anterior horn cells, dorsal root ganglia and in skeletal muscle cells. Other developing tissues and organs that showed evidence of fetuin synthesis and presence of the protein included mesenchyme, kidney, adrenal, developing bone, gut, lung and heart. In the immature liver (E30–40) there was a strong signal for fetuin mRNA in hepatocytes and also in numerous haemopoietic cells; the proportion of these latter cells that was positive for fetuin mRNA increased between E30 and E40. Only some hepatocytes and a proportion of the haemopoietic stem cells were immunoreactive for fetuin itself at E30–40; immunoreactive hepatocytes were more frequently observed in the more mature outer regions of the developing liver. Lung and gut contained scattered fetuin-positive epithelial cells, especially at E30; a weak fetuin mRNA signal could be detected above background in many of these cells up to E40, but not at E60–E115 or in the adult. Particularly at E30 to E40, mesenchymal tissue both within organs such as the gut and lung and around forming bone and skeletal muscle contained cells that were positive for fetuin mRNA. Mesenchyme at these ages was also very strongly stained for fetuin protein, much of which may reflect fetuin in tissue extracellular spaces and be derived from the high concentration in plasma. By E80 fetuin mRNA was mainly present in the liver and the CNS; staining of the muscle tissue was becoming less pronounced. However in developing bone tissue, staining of chondrocytes for fetuin mRNA was still prominent in older (E80) fetuses; there was also fetuin protein staining of chondrocytes at the growing surfaces of bones and in bone marrow at this age. In the adult, weak immunocytochemical staining for fetuin itself was present in hepatocytes, but the mRNA signal was barely above the threshold limit of detection. Other tissues in the adult were generally negative for both fetuin mRNA and fetuin, except that fetuin could generally be detected immunocytochemically in precipitated plasma within vessels in many tissues and in their interstitial spaces. The highest levels of fetuin mRNA, as demonstrated by RT-PCR, were detected in E40 and E60 liver followed by E40 muscle. The very low level of fetuin mRNA in adult liver, evident from in situ hybridisation, was confirmed by RT-PCR (about 0.1% of that at E60). These results show that in many tissues in which fetuin could be demonstrated immunocytochemically, its presence is likely to be due to synthesis in situ. However in some instances (e.g. gut and mesenchymal tissue) fetuin probably originates predominantly by uptake from plasma or extracellular fluid. The functional significance of the presence of fetuin in different tissues during their development is considered.
Similar content being viewed by others
References
Billington WD, Wild AE (1979) Maternal immunological factors in embryonic and post-natal development. In: Newth DR, Balls M (eds) Maternal effects in development. (BSDB Symposium 4) Cambridge University Press, Cambridge, pp 321–350
Brown WM, Christie DL, Dziegielewska KM, Nawratil P, Saunders NR, Müller-Esterl W (1992a) The nucleotide and deduced amino acid structure of sheep and pig fetuin. Common structural features of the mammalian fetuin family. Eur J Biochem 205:321–331
Brown WM, Møllgård K, Saunders NR, Dziegielewska KM (1992b) Fetuin an old friend revisited. Bioessays 14:749–755
Cavanagh ME, Warren A (1985) Distribution of native and foreign albumin injected into lateral ventricles of prenatal and neonatal rat. Anat Embryol 172:345–351
Christie DL, Dziegielewska KM, Hill RM, Saunders NR (1987) Fetuin: the bovine homologue of human α2HS glycoprotein. FEBS Lett 214:45–49
Dziegielewska KM, Evans CAN, Malinowska D, Møllgård K, Reynolds JM, Reynolds ML, Saunders NR (1979) Studies of the development of brain barrier systems to lipid insoluble molecules in foetal sheep. J Physiol 292:207–231
Dziegielewska KM, Evans CAN, Fossan G, Lorscheider FL, Malinowska DH, Mollgard K, Reynolds ML, Saunders NR, Wilkinson S (1980) Proteins in cerebrospinal fluid and plasma of fetal sheep during development. J Physiol 300:441–445
Dziegielewska KM, Møllgård K, Reynolds ML, Saunders NR (1987) A fetuin-related glycoprotein (α2HS) in human embryonic and fetal development. Cell Tissue Res 248:33–41
Dziegielewska KM, Brown WM, Casey SJ, Christie DL, Foreman RC, Hill RM, Saunders NR (1990) The complete cDNA and amino acid sequence of bovine fetuin. Its homology with α2HS glycoprotein and relation to other members of the cystain superfamily. J Biol Chem 265:4354–4357
Dziegielewska KM, Møllgård K, Saunders NR, Reader M (1993) Fetuin synthesis in cells of the immature neocortex. J Neurocytol 22:266–272
Jacobsen M, Møllgård K, Reynolds ML, Saunders NR (1983) The choroid plexus in fetal sheep during development with special reference to intracellular plasma protein. Dev Brain Res 8:77–88
Møllgård K, Balslev Y (1989) The subcellular distribution of transferrin in rat choroid plexus studied with immunogold labelling of ultracryosections. Histochem J 21:441–448
Møllgård K, Reynolds ML, Jacobsen M, Dziegielewska KM, Saunders NR (1984) Differential immunocytochemical staining for fetuin and transferrin in the developing cortical plate. J Neurocytol 13:497–502
Reynolds ML, Møllgård K (1985) The distribution of plasma proteins in the neocortex and early allocortex of the developing sheep brain, Anat Embryol 171:41–60
Reynolds ML, Møllgård K, Saunders NR (1983) The distribution of plasma proteins during early embryonic development in the sheep. Anat Embryol 168:227–240
Reynolds ML, Sarantis MEP, Lorscheider FL, Saunders NR (1987) Fetuin as a marker of cortical plate cells in the fetal cow neocortex: a comparison of the distribution of fetuin, α2HS glycoprotein, α-fetoprotein and albumin during early development. Anat Embryol 175:355–363
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Earlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Saunders NR (1984) Plasma proteins and fetal brain development In: Duprat AM, Kato AC, Weber M (eds) The role of cell interactions in early neurogenesis. (NATO Advanced Study Institute) Plenum, New York
Saunders NR, Habgood MD, Ward RA, Reynolds ML (1992) Origin and fate of fetuin-containing neurons in the developing neocortex of the fetal sheep. Anat Embryol 186:477–486
Sheardown SA (1992) A simple method for affinity purification and PCR amplification of poly(A)+ mRNA. Trends Genet 8:121
Thorbecke GJ, Huriemann J, Silverstein AM (1967) Production of fetuin and other serum proteins by fetal sheep liver in vitro. Proc Soc Exp Biol 126:816–819
White H, Totty N, Panayotou G (1993) Haemonectin, a granulocytic-cell binding protein, is related to the plasma glycoprotein fetuin. Eur J Biochem 213:523–528
Yang F, Schwartz Z, Swain LD, Lee C-C, Bowman BH, Boyan BD (1991) α2HS glycoprotein: expression in chondrocytes and augmentation of alkaline phosphatase and phospholipase A2 activity. Bone 12:7–15
Yang F, Chen Z-L, Bergeron JM, Cupples RL, Friedrichs WE (1992) Human α2HS glycoprotein/bovine fetuin homologue in mice: identification and developmental regulation of the gene. Biocheim Biophys Acta 1130:149–156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saunders, N.R., Deal, A., Dziegielewska, K.M. et al. Expression and distribution of fetuin in the developing sheep fetus. Histochemistry 102, 457–475 (1994). https://doi.org/10.1007/BF00269578
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00269578