Abstract
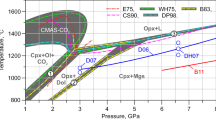
The stability field of the end-member scapolite meionite was determined in piston-cylinder apparatus. Meionite has very high thermal stability at high pressures, exceeding 1500° C at 20 kbar. Below 6 kbar and 1270 ° C scapolite breakdown is subsolidus, to an-orthite + gehlenite + wollastonite + CO2, with a slope of 20 bars/degree. An extrapolation of existing thermodynamic data for CO2 permits calculation of ΔG oF =-2384.5 kcal/mol for meionite at 1270 ° C, very close to the value for 3 anorthite + calcite. Above 1270 ° C, scapolite begins to melt to An+Geh+Liq+CO2, and as pressure increases the melting curve steepens, the Geh and An being progressively replaced by Liq+corundum with Al in 6-coordination. At pressures >25kbar dp/dt becomes negative, corundum is the only crystalline product, and CO2 bubbles disappear from the quenched glass, indicating a solubility of CO2 under these conditions of about 5 wt. percent in the liquid.
The subsolidus breakdown of meionite at high pressures to grossularite + kyanite + quartz + calcite nearly coincides with the upper pressure limits of anorthite. Thus scapolite is essentially limited to crustal rocks. In view of its great thermal stability, meionite can play a role as a primary mineral in deep-seated basic or intermediate magmatic processes. It is also likely that CO2 coming from the earth's interior will be captured by reaction with plagioclase and clinopyroxene. Scapolite has been noted in basic granulite inclusions from basaltic pipes in three continents. It seems probable that scapolite acts as a major storage site for CO2 in the deep crust.
Similar content being viewed by others
References
Bobrievich, A. P., Sobolev, U. S.: Eclogitization of the pyroxene crystalline schists of the Archean complex (in Russian). Zap. Vses. Mineralog. Obshchestva, V. Ser. 86, 3–17 (1957)
Borchert, H.: Zur Geochimie des Kohlenstoffs. Geochim. Cosmochim. Acta 2, 62–75 (1951)
Brögger, W. C.: On several Archaean rocks from the south coast of Norway. Pt. I. Skrifter Norske Videnskaps-Akad. Oslo, Mat.-Naturv. Kl, No. 8 (1933)
Burnham, C. W.: Lattice constant refinement. Ann. Rept. of the Dir. of the Geophysical Lab., Carnegie Inst. Wash. Yearbook 61, 132–135 (1962)
Cox, K. G., Gurney, J. J., Harte, B.: Xenoliths from the Matsoku pipe. In: Lesotho kimberlites. P. H. Nixon, ed., Lesotho National Development Corp. Maseru, 76–100 (1973)
Dawson, J. B.: Carbonate tuff cones in northern Tanganyika. Geol. Mag. 101, 129–137 (1964)
Dawson, J. B.: Recent researches on kimberlite and diamond geology. Economic Geol. 63, 504–511 (1968)
Dawson, J. B.: Advances in kimberlite geology. Earth-Sci. Rev. 7, 187–214 (1971)
Eugster, H. P., Prostka, H. J.: Synthetic scapolites. Bull. Geol. Soc. Am. 71, 1858 (1960) (Abstract)
Eugster, H. P., Prostka, H. J., Appleman, D. E.: Unit-cell dimensions of natural and synthetic scapolites. Science 137, 853–854 (1962)
Gibbs, G. V., Bloss, F. D.: Indexed powder diffraction data for scapolite. Am. Mineralogist 46, 1493–1497 (1961)
Goldsmith, J. R., Heard, H. C.: Subsolidus phase relations in the system CaCO3-MgCO3. J. Geol. 69, 45–74 (1961)
Hariya, Y., Kennedy, G. C.: Equilibrium study of anorthite under high pressure and temperature. Am. J. Sci. 266, 193–203 (1968)
Irving, A. J.: Geochemical and high pressure experimental studies of garnet pyroxenite and pyroxene granulite xenoliths from the Delegate basaltic pipes, Australia. J. Petrol. 15, 1–40 (1974)
Kushiro, I.: Wollastonite-pseudowollastonite inversion. Ann. Rept. of the Dir. of the Geopysical Lab., Carnegie Inst. Wash. Yearbook 23, 83–84 (1964)
Kushiro, I., Yoder, H. S.: Anorthite-forsterite and anorthite-enstatite reactions and their bearing on the basalt-eclogite transformation. J. Petrol. 7, 337–362 (1966)
Lovering, J. F., White, A. J. R.: The significance of primary scapolite in granulitic inclusions from deep-seated pipes. J. Petrol. 5, 195–218 (1964)
Lovering, J. P., White, A. J. R.: Granulitic and eclogitic inclusions from basic pipes at Delegate, Australia. Contrib. Mineral. Petrol. 21, 9–52 (1969)
Millhollen, G. L.: Synthesis of scapolite under magmatic conditions. Am. Mineralogist 59, 618–620 (1974)
Misch, P.: Stable association wollastonite-anorthite and other calc-silicate assemblages in amphibolite facies crystalline schists of Nanga Parbat, northwest Himalayas. Beitr. Mineral. Petrog. 10, 315–356 (1964)
Robie, R. A., Waldbaum, D. R.: Thermodynamic properties of minerals and related substances at 198.15 K (25.0° C) and one atmosphere (1.013 bars) pressure and at higher temperatures. U. S. Geol. Surv. Bull. 1259, 256 pp. (1968)
Ronov, A. B., Yaroshevskiy, A. A.: Chemical structure of the Earth's crust. Geochemistry, 1041-1066 (1967), trans. from Geokhimiya 11, 1285–1309 (1967)
Sharp, W. E.: The thermodynamic functions for carbon dioxide in the range 40 to 1000° C and 1 to 1400 bars. Univ. of Calif. Lawrence Rad. Lab. Publ. No. 7168-TID-4500, 52 pp. (1962)
Shaw, D. M.: The geochemistry of scapolite. Part I. Previous work and general mineralogy. J. Petrol. 1, 218–260 (1960a)
Shaw, D. M.: The geochemistry of scapolite. Part II. Trace elements, petrology, and general geochemistry. J. Petrol. 1, 261–285 (1960b)
Sobolev, V. S. (editor): The diamond deposits of Yakutia. Moscow, 527 pp. (1959)
Touret, J.: Le faciès granulite en Norvège meridionale. Pts. I and II. Lithos 4, 239–249, 423–436 (1971)
Touret, J.: Facies granulite et fluids carboniques. Ann. Soc. Geol. Belg. (in press)
Verhoogen, J.: Les pipes de kimberlite du Katanga. Ann. Services Mines, Comité Special du Katanga 9, 3–46 (1938)
Wenk, E.: Plagioklas als indexmineral in den zentralalpen. Schweiz. Mineral. Petrog. Mitt. 42, 139–152 (1962)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Newton, R.C., Goldsmith, J.R. Stability of the scapolite meionite (3CaAl2Si2O2 · CaCO3) at high pressures and storage of CO2 in the deep crust. Contrib. Mineral. Petrol. 49, 49–62 (1975). https://doi.org/10.1007/BF00371078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371078