Abstract
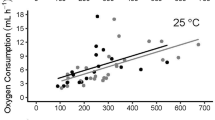
We measured metabolic rates at 15 and 25°C in 42 helodermatid lizards ranging in mass from 26 to 1616 g. No consistent repeatable daily rhythms of metabolism were detected. There were no significant differences in metabolic rates between the two species of Heloderma. The temperature coefficient for metabolism (Q 10) was 3.0 between 15 and 25°C. The mass exponent for helodermatids (0.69) differed significantly from the among-species mass exponent of 0.80 for all squamates combined. However, adult Heloderma had a mass exponent of 0.80. Rates of metabolism of adult helodermatids were lower than those of other squamate reptiles, and at 15°C periods of apnea contributed to a further reduction in metabolic rate. Our finding that helodermatids have low SMRs supports the hypothesis that ecology is important in influencing metabolic rate, and that “reclusive” squamates have lower rates of metabolism than do nonreclusive species.
Similar content being viewed by others
Abbreviations
- BM:
-
body mass
- ME:
-
mass exponent
- MR:
-
metabolic rate(s)
- RMR:
-
resting metabolic rate(s)
- SMR:
-
standard metabolic rate(s)
- T a :
-
ambient temperature
- T b :
-
body temperature
References
Al-Sadoon MK (1991) Metabolic rate-temperature curves of the horned viper, Cerastes cerastes gasperetti, the miola snake, Malpolon moilensis, and the adder, Vipera berus. Comp Biochem Physiol 99A:119–122
Al-Sadoon MK, Abdo NM (1992) Temperature and body mass effects on the metabolic rate of Acanthodactylus schmidti Weigmann (Reptilia: lacertidae). J Arid Environ 21:351–362
Anderson RA, Karasov WH (1981) Contrasts in energy intake and expenditure in sit-and-wait and widely foraging lizards. Oecologia 49:67–72
Andrews RM, Pough FH (1985) Metabolism of squamate reptiles: allometric and ecological relationships. Physiol Zool 58:214–231
Auffenberg W (1981) The behavioral ecology of the komodo monitor. University of Florida Press, Gainesville, USA
Auffenberg W (1988) Gray's monitor lizard. University of Florida Press, Gainesville, USA
Bartholomew GA, Tucker VA (1964) Size, body temperature, thermal conductance, oxygen consumption, and heart rate in Australian varanid lizards. Physiol Zool 37:341–354
Beaupre SJ (1993) An ecological study of oxygen consumption in the mottled rock rattlesnake, Crotalus lepidus lepidus, and the black-tailed rattlesnake, Crotalus molossus molossus, from two populations. Physiol Zool 66:437–454
Beck DD (1990) Ecology and behavior of the Gila monster in southwestern Utah. J Herpetol 24:54–68
Beck DD (1991) Physiological and behavioral consequences of reptilian life in the slow lane: ecology of beaded lizards and rattlesnakes. PhD dissertation, University of Arizona, Tucson, USA
Beck DD, Lowe CH (1991) Ecology of the beaded lizard Heloderma horridum, in a tropical dry forest in Jalisco, Mexico. J Herpetol 25:395–406
Beck DD, Ramirez-Bautista A (1991) Combat behavior of the beaded lizard Heloderma h. horridum in Jalisco, Mexico. J Herpetol 25:481–484
Bennett AF (1982) The energetics of reptilian activity. In: Gans C, Pough FH (eds) Biology of the Reptilia, vol 13. Academic Press, New York, pp 155–199
Bennett AF, Dawson WR (1976) Metabolism. In: Gans C, Dawson WR (eds) Biology of the Reptilia, vol 5. Academic Press, New York, pp 127–223
Bennett AF, Ruben JA (1979) Endothermy and activity in vertebrates. Science 206:649–654
Bogert CM, Martin del Campo R (1956) The Gila monster and its allies. Bull Am Mus Nat Hist 109:1–238
Brody S (1945) Bioenergetics and growth. Reinhold, New York
Campbell JA, Lamar WL (1989) The venomous reptiles of Latin America. Cornell University Press, Ithaca, New York, USA
Chappell MA, Ellis TM (1987) Resting metabolic rates in boid snakes: allometric relationships and temperature effects. J Comp Physiol B 157:227–235
Cragg PA (1978) Oxygen consumption in the lizard genus Lacerta in relation to diel variation, maximum activity and body weight. J Exp Biol 77:33–56
Dryden G, Green B, King D, Losos J (1991) Water and energy turnover in a small monitor lizard, Varanus acanthurus. Austr Wildl Res 17:641–646
Dunson WA, Bramham CR (1981) Evaporative water loss and oxygen consumption of three small lizards from the Florida Keys: Sphaerodactylus cinereus, S. notatus, and Anolis sagrei. Physiol Zool 54:253–259
Estes R, Queiroz K de, Gauthier J (1988) Phylogenetic relationships within squamata. In: Estes R, Pregill G (eds) Phylogenetic relationships of the lizard families: essays commemorating Charles L. Camp. Stanford University Press, Stanford, pp 119–281
Feder ME (1987) Effect of thermal acclimation on locomotor energetics and locomotor performance in a tropical salamander, Bolitoglossa subpalmata. Physiol Zool 60:18–26
Feder ME, Feder JH (1981) Diel variation of oxygen consumption in three species of Philippine gekkonid lizards. Copeia 1981:204–209
Fusari M (1984) Temperature responses of standard aerobic metabolism by the California legless lizard, Anniellapulchra. Comp Biochem Physiol 77A:97–102
Garland T Jr (1993) Locomotor performance and activity metabolism of Cnemidophorus tigris in relation to natural behavior. In: Wright JW, Vitt LJ (eds) Biology of whiptail lizards (genus Cnemidophorus). Oklahoma Mus Nat Hist, Norman, Oklahoma, USA, pp 163–210
Hill RW (1972) Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J Appl Physiol 33:261–263
Heusner AA (1982) Energy metabolism and body size. I. Is the 0.75 mass exponent of Kleiber's equation a statistical artifact? Respir Physiol 48:1–12
John-Alder HB, Lowe CH, Bennett AF (1983) Thermal dependence of locomotory energetics and aerobic capacity of the gila monster (Heloderma suspectum). J Comp Physiol 151:119–126
John-Alder HB, Garland T Jr, Bennett AF (1986) Locomotory capacities, oxygen consumption, and the cost of locomotion of the shingle-back lizard (Trachydosaurus rugosus). Physiol Zool 59:523–531
Kamel S, Gatten RE Jr (1983) Aerobic and anaerobic activity metabolism of limbless and fossorial reptiles. Physiol Zool 56:419–429
Losos JB, Greene HW (1988) Ecological and evolutionary implications of diet in monitor lizards. Biol J Linn Soc 35:379–407
Loumbourdis NS, Hailey A (1985) Activity metabolism of the lizard Agama stellio stellio. Comp Biochem Physiol 82A:687–691
Lowe CH, Hinds DS, Lardner PJ, Justice KE (1967) Natural freerunning period in vertebrate animal populations. Science 155:531–534
Lowe CH, Schwalbe CR, Johnson TB (1986) The venomous reptiles of Arizona. Arizona Game and Fish Dept, Phoenix, Arizona, USA
Mautz WJ (1979) The metabolism of reclusive lizards, the Xantusiidae. Copeia 1979:577–584
Nagy KA, Huey RA, Bennett AF (1984) Field energetics and foraging mode of Kalahari lacertid lizards. Ecology 65:588–596
Pough FH, Andrews RM (1984) Individual and sibling-group variation in metabolism of lizards: the aerobic capacity model for the origin of endothermy. Comp Biochem Physiol 79A:415–419
Pregill GK, Gauthier JA, Greene HW (1986) The evolution of helodermatid squamates, with description of a new taxon and an overview of Varanoidea. Trans San Diego Soc Nat Hist 21:167–202
Putnam RW, Murphy RW (1982) Low metabolic rate in a nocturnal desert lizard, Anarbylus switaki Murphy (Sauria: Gekkonidae). Comp Biochem Physiol 71A:119–123
Roberts LA (1968) Oxygen consumption in the lizard Uta stansburiana. Ecology 49:809–819
Secor SM (1992) Activities and energetics of a sit-and-wait snake, Crotalus cerastes. PhD dissertation, University of California, Los Angeles, USA
Secor SM, Nagy KA (1994) Energetic correlates of foraging mode for the snakes Crotalus cerastes and Masticophis flagellum. Ecology (in press)
Sokal RR, Rolf FJ (1981) Biometry, 2nd edn. Freeman, San Francisco
Schmidt-Nielsen K (1983) Animal physiology: adaptation and environment, 3rd edn. Cambridge University Press, New York
Taigen RL (1983) Activity metabolism of anuran amphibians: implications for the origin of endothermy. Am Nat 121:94–109
Thompson GC, Withers PC (1992) Effects of body mass and temperature on standard metabolic rates for two Australian varanid lizards (Varanus gouldii and V. panoptes). Copeia 1992:343–350
Withers, PC (1981) Physiological correlates of limblessness and fossoriality in scincid lizards. Copeia 1981:197–204
Wood SC, Johansen K, Glass ML, Maloiy GMO (1978) Aerobic metabolism of the lizard Varanus exanthematicus: effects of activity, temperature, and size. J Comp Physiol 127:331–336
Zari TA (1991) The influence of body mass and temperature on the standard metabolic rate of the herbivorous desert lizard, Uromastyx microlepis. J Therm Biol 16:129–134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beck, D.D., Lowe, C.H. Resting metabolism of helodermatid lizards: allometric and ecological relationships. J Comp Physiol B 164, 124–129 (1994). https://doi.org/10.1007/BF00301653
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00301653