Abstract
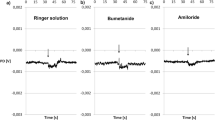
The dorsal skin of the leech Hirudo medicinalis was used for electrophysiological measurements performed in Ussing chambers. The leech skin is a tight epithelium (transepithelial resistance = 10.5±0.5 kΩ· cm-2) with an initial short-circuit current of 29.0±2.9 μA·cm-2. Removal of Na+ from the apical bath medium reduced short-circuit current about 55%. Ouabain (50μmol·l-1) added to the basolateral solution, depressed the short-circuit current completely. The Na+ current saturated at a concentration of 90 mmol Na+·l-1 in the apical solution (K M=11.2±1.8 mmol·l-1). Amiloride (100 μmol·l-1) on the apical side inhibited ca. 40% of the Na+ current and indicated the presence of Na+ channels. The dependence of Na+ current on the amiloride concentration followed Michaclis-Menten kinetics (K i=2.9±0.4 μmol·l-1). The amiloride analogue benzamil had a higher affinity to the Na+ channel (K i=0.7±0.2 μmol·l-1). Thus, Na+ channels in leech integument are less sensitive to amiloride than channels known from vertebrate epithelia. With 20 mmol Na+·l-1 in the mucosal solution the tissue showed an optimum amiloride-inhibitable current, and the amiloride-sensitive current under this condition was 86.8±2.3% of total short-circuit current. Higher Na+ concentrations lead to a decrease in amiloride-blockade short-circuit current. Sitmulation of the tissue with cyclic adenosine monophosphate (100 μmol·l-1) and isobutylmethylxanthine (1 mmol·l-1) nearly doubled short-circuit current and increased amiloride-sensitive Na+ currents by 50%. By current fluctuation analysis we estimated single Na+ channel current (2.7±0.9 pA) and Na+ channel density (3.6±0.6 channels·μm-2) under control conditions. After cyclic adenosine monophosphate stimulation Na+ channel density increased to 5.4±1.1 channels·μm-2, whereas single Na+ channel current showed no significant change (1.9±0.2 pA). These data present a detailed investigation of an invertebrate epithelial Na+ channel, and show the similarities and differences to vertebrate Na+ channels. Whereas the channel properties are different from the classical vertebrate Na+ channel, the regulation by cyclic adenosine monophosphate seems similar. Stimulation of Na+ uptake by cyclic adenosine monophosphate is mediated by an increasing number of Na+ channels.
Similar content being viewed by others
Abbreviations
- α:
-
slope of the background noise component
- ADH:
-
antidiuretic hormone
- cAMP:
-
cyclic adenosine monophosphate
- f :
-
frequency
- f c :
-
coner frequency of the Lorentzian noise component
- Hepes:
-
N-hydroxyethylpiperazine-N′-ethanesulphonic acid
- BMX:
-
isobutyl-methylxanthine
- i Na :
-
single Na+ channel current
- I Na :
-
max, maximal inhibitable Na+ current
- I SC :
-
short circuit current
- K i :
-
half maximal blocker concentration
- K M :
-
Michaelis constandard error of the mean
- S (f) :
-
power density of the Lorentzian noise component
- S 0 :
-
plateau value of the Lorentzian noise component
- TMA:
-
tetramethylammonium
- Trizma:
-
TRIS-hydroxymethyl-amino-methane
- V max :
-
maximal reaction velocity
- V T :
-
transepithelial potential
- K :
-
half maximal blocker concentration
References
Abramcheck FJ, Van Driessche W, Helman SI (1985) Autoregulation of apical membrane Na permeability of tight epithelia. J Gen Physiol 85:555–582
Benos DJ (1982) Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol 242: C131-C145
Benos DJ, Simon SA, Mandel LJ, Cala PM (1976) Effects of amiloride and some of its analogues on cation transport in isolated frog skin and thin lipid membranes. J Gen Physiol 68:43–63
Bentley PJ (1968) Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol (London) 195:317–330
Catarsi S, Garcia-Gil M, Traina G, Brunelli M (1990) Seasonal variation of serotonin content and non-associative learning of swim induction in the leech Hirudo medicinalis. J Comp Physiol A 167:469–474
Chase H (1984) Does calcium couple the apical and basolateral membrane permeabilities in epithelia? Am J Physiol 247:F869-F876
Clauss W, Dürr JE, Guth D, Skadhauge E (1987) Effects of adrenal steroids on Na transport in the lower intestine (coprodeum) of the hen. J Membr Biol 96:141–152
Cobbold PH (1971) Uptake of sodium and potassium by the leech, Hirudo medicinalis. J Physiol (London) 219:10P
Dannenmaier B, Heinke B, Weber W-M, Clauss W (1991a) Amiloride-sensitive Na+ channels in the dorsal skin of Hirudo medicinalis. Verh Dtsch Zool Ges 84:402–403
Dannenmaier B, Weber W-M, Clauss W (1991b) Characterization of an amiloride-sensitive Na+ channel in an invertebrate (Hirudo medicinalis). Pflügers Arch 419 [Suppl]:R103
Garty H, Benos DJ (1988) Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev 68:309–373
Garty H, Edelman IS (1983) Amiloride-sensitive trypsinization of acpical sodium channels. J Gen Physiol 81:785–803
Hamilton KL, Eaton D (1985) Single-channel recordings from amiloride-sensitive epithelial channel. Am J Physiol 249:C200-C207
Helman SI, Cox TC, Van Driessche W (1983) Hormonal control of apical membrane Na+ transport in epithelia. Studies with fluctuation analysis. J Gen Physiol 82:201–220
Kirschner LB, Greenwald L, Kerstetter TH (1973) Effect of amiloride on sodium transport across body surfaces of freshwater animals. Am J Physiol 224:832–837
Kleymann TR, Cragoe EJ (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21
Krattenmacher R, Clauss W (1988) Electrophysiological analysis of sodium-transport in the colon of the frog (Rana esculenta). Pflügers Arch 411:606–612
Krattenmacher R, Clauss W (1989) Autoregulation of apical sodium entry in the colon of the frog (Rana esculenta). Comp Biochem Physiol 93A:593–596
Lent CM, Dickinson MH (1988) Neurobiologie des Freßverhaltens von Blutegeln. Spektrum der Wissenschaften 8:104–110
Li H-Y, Lindermann B (1983) Chemical stimulation of Na+ transport through amiloride-blockable channels of frog epithelium. J Membr Biol 75:179–192
Lindemann B (1984) Fluctuation analysis of sodium channels in epithelia. Annu Rev Physiol 46:497–515
Onken H, Riestenpatt S, Zeiske W (1991) Na+ absorption across the gill epithelium of the chinese crab: characteristics of the apical Na+ channel. Verh Dtsch Zool Ges 84:417–418
Orloff J, Handler JS (1962) The similarity of effects of vassopressin, adenosine 3–5 phosphate (cAMP) and theophylline on the toad bladder. J Clin Invest 41:702–709
Palmer LG (1987) Ion selectivity of epithelial Na+ channels. J Membr Biol 96:97–106
Prusch RD, Otter T (1976) Annelid transepithelial ion transport. Comp Biochem Physiol 57A:87–92
Schwarz HJ, Graszynski K (1990) Characterization of the Na+ transport from posterior gills of the chinese crab Eriocheir sinensis using the voltage clamp technique. Verh Dtsch Zool Ges 83:555–556
Smith PR, Benos DJ (1991) Epithelial Na+ channels. Annu Rev Physiol 53:509–530
Van Driessche W, Erlij D (1983) Noise analysis of inward and outward Na+ currents across the apical border of ouabain-treated frog skin. Pflügers Arch 398:179–188
Van Driessche W, Lindemann B (1979) Concentration dependence of currents through single-sodium-selective pores in frog skin. Nature (London) 282:519–520
Van Driessche W, Zeiske W (1985) Ionic channels in epithelial cell membranes. Physiol Rev 65:833–903
Zerbst-Boroffka I (1984) The homeostatic function of integument and nephridia in annelids. In: Pequeux A, Gilles R, Bolis L (eds) Osmoregulation in estuarine and marine animals. Springer, Heidelberg, pp 4–15
Zerbst-Boroffka I, Wenning A (1986) Mechanisms of regulatory salt and water excretion in the leech, Hirudo medicinalis L. Zool Beitr NF 30:359–377
Zerbst-Boroffka I, Kollmann R, Hildebrandt JP (1983) Mechanisms of regulatory salt and water excretion in the leech, Hirudo medicinalis. Verh Dtsch Zool Ges 76:243
Zeiske W, Onken H, Schwarz HJ, Graszynski K (1992) Invertebrate epithelial Na+ channels: amilorid-induced current-noise in crab gill. Biochim Biophys Acta 1105:245–252
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weber, W.M., Dannenmaier, B. & Clauss, W. Ion transport across leech integument. J Comp Physiol B 163, 153–159 (1993). https://doi.org/10.1007/BF00263601
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00263601