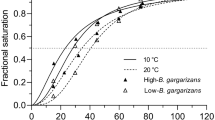
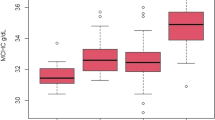
Summary
To estimate the advantage of the small red blood cells (RBC) of high-altitude camelids for O2 transfer, the kinetics of O2 uptake into and release from the RBC obtained from llama, vicuña and alpaca were investigated at 37°C with a stopped-flow technique. O2 transfer conductance of RBC (G) was estimated from the rate of O2 saturation change and the corresponding O2 pressure difference between medium and hemoglobin. For comparison, O2 kinetics for the RBC of a lowaltitude camelid (dromedary camel) and the pygmy goat were determined and previously measured values for human RBC were used. O2 transfer of RBC was found to be strongly influenced by extracellular diffusion, except with O2 release into dithionite solutions of sufficiently high concentration (>30 mM). TheG values measured in these ‘standard’ conditions,G st (in mmol · min−1 · Torr−1 · (ml RBC)−1) were: high-altitude camelids, 0.58 (averaged for llama, alpaca and vicuña since there were no significant interspecific differences); camel 0.42; goat, 0.42; man, 0.39. The differences can in part be attributed to expected effects of the size and shape of the RBC (volume, surface area, mean thickness), as well as to the intracellular O2 diffusivity which depends on the concentration of cellular hemoglobin. The highG st of RBC of highaltitude camelids may be considered to enhance O2 transfer in lungs and tissues. But the O2 transfer conductance of blood, θ, equal toG st multiplied by hematocrit (in mmol · min−1 · Torr−1 · (ml blood)−1), was only slightly higher as compared to other species: 0.20 (llama, alpaca, vicuña), 0.14 (camel), 0.18 (goat), 0.17 (man).
Similar content being viewed by others
Abbreviations
- DPG :
-
2,3-diphosphoglycerate
- G :
-
conductance
- Hb :
-
hemoglobin
- RBC :
-
red blood cells
- \(S_{O_2 } \) :
-
percent saturation of hemoglobin
References
Bartels H, Hilpert P, Barbey K, Betke K, Riegel K, Lang EM, Metcalfe J (1963) Respiratory functions of blood of the yak, llama, camel, Dybowski deer, and African elephant. Am J Physiol 205:331–336
Bouverot P (1985) Adaptation to altitude-hypoxia in vertebrates. Springer, Berlin Heidelberg New York
Chiodi H (1970/71) Comparative study of the blood gas transport in high altitude and sea level camelidae and goats. Respir Physiol 11:84–93
Coin JT, Olson JS (1979) The rate of oxygen uptake by human red blood cells. J Biol Chem 254:1178–1190
Drabkin DL, Austin JH (1935) Spectrophotometric studies. II. Preparations from washed blood cells, nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 112:51–65
Ericson A, de Verdier CH (1972) A modified method for the determination of 2,3-diphosphoglycerate in erythrocytes. Scand J Clin Lab Invest 29:85–90
Evelyn KA, Malloy HT (1938) Microdetermination of oxyhemoglobin, methemoglobin, and sulfhemoglobin in a single sample of blood. J Biol Chem 126:655–662
Hilpert P, Fleischmann RG, Kempe D, Bartels H (1963) The Bohr effect related to blood and erythrocyte pH. Am J Physiol 205:337–340
Holland RAB, Forster RE (1966) The effect of size and red cells on the kinetics of their oxygen uptake. J Gen Physiol 49:727–742
Holland RAB, Shibata H, Scheid P, Piiper J (1985) Kinetics of O2 uptake and release by red cells in stopped-flow apparatus: effects of unstirred layer. Respir Physiol 59:71–91
Huxley VH, Kutchai H (1981) The effect of the red cell membrane and a diffusion boundary layer on the rate of oxygen uptake by human erythrocytes. J Physiol 316:75–88
Huxley VH, Kutchai H (1983) Effect of diffusion boundary layers on the initial uptake of O2 by the cells. Theory versus experiment. Microvasc Res 26:89–107
Jones DA (1979) The importance of surface area/volume ratio to the rate of oxygen uptake by red cells. J Gen Physiol 74:643–646
Jürgens KD, Bartels H, Bartels R (1981) Blood oxygen transport and organ weights of small bats and small non-flying mammals. Respir Physiol 45:243–260
Kreuzer F (1970) Facilitated diffusion of oxygen and its possible significance: a review. Respir Physiol 9:1–30
Middleman S (1972) Transport phenomena in the cardiovascular system. Chap 2. Wiley, New York
Miyamoto Y, Moll W (1972) The diameter of red blood cells when flowing through a rapid reaction apparatus. Respir Physiol 16:259–266
Moll W (1968/69) The influence of hemoglobin diffusion on oxygen uptake and release by red cells. Respir Physiol 6:1–15
Monge C, Whittembury J (1976) High altitude adaptation in the whole animal. In: Bligh J, Cloudsley-Thompson JL, Macdonald AG (eds) Environmental physiology of animals. Blackwell, Oxford, pp 287–308
Reynafarje C (1966) Iron metabolism during and after altitude exposure in man and in adapted animals (camelids). Fed Proc 25:1240–1242
Rice SA (1980) Hydrodynamic and diffusion considerations of rapid-mix experiments with red blood cells. Biophys J 29:65–78
Riegel K, Bartels H, Kleihauer E, Lang EM, Metcalfe J (1966) Comparative studies of the respiratory functions of mammalian blood. I. Gorilla, chimpanzee and orangutan. Respir Physiol 1:138–144
Scatchard G, Batchelder AC, Brown A (1946) Preparation and properties of serum and plasma proteins. VI. Osmotic equilibria in solutions of serum albumin and sodium chloride. J Am Chem Soc 68:2320–2329
Sendroy J, Dillon RT, Van Slyke DD (1934) Studies of gas and electrolyte equilibria in blood. J Biol Chem 105:597–632
Smith JE, Mohandas N, Shohet SB (1979) Variability in erythrocyte deformability among various mammals. Am J Physiol 236:H725-H730
Vandegriff KD, Olson JS (1984) Morphological and physiological factors affecting oxygen uptake and release by red blood cells. J Biol Chem 259:12619–12627
Yamaguchi K, Nguyen-Phu D, Scheid P, Piiper J (1985) Kinetics of O2 uptake and release by human red blood cells studied by a stopped-flow technique. J Appl Physiol 58:1215–1224
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaguchi, K., Jürgens, K.D., Bartels, H. et al. Oxygen transfer properties and dimensions of red blood cells in high-altitude camelids, dromedary camel and goat. J Comp Physiol B 157, 1–9 (1987). https://doi.org/10.1007/BF00702722
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00702722