Abstract
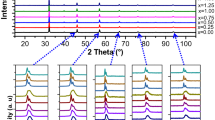
Electronic absorption spectra have been measured at room temperature and pressure for polycrystalline samples of (Mg, Fe)SiO3 silicate perovskites synthesized by multi-anvil device. One strong near-infrared band at about 7000 cm-1 and several weak bands in the visible region were found. The near-infrared band at 7000 cm-1 is assigned to a spin-allowed transition of Fe2+ at the 8–12 coordinated site in perovskite. However, definite assignments of the weak bands in the visible region are difficult because of their low intensities and the scattering effect at the gain boundaries. Crystal field calculations for Fe2+ at different sites in perovskite have been carried out based on the crystal structure data. The results agree with the assignment of Fe2+ to the 8–12 coordinated site in perovskite. Crystal field stabilization energy of Fe2+ with coordination number of 8 in perovskite is 3332 cm-1 which is small compared to the octahedral site of magnesiowüstite (4320 cm-1), another important lower-mantle mineral.
Similar content being viewed by others
References
Burns RG (1985) Thermodynamic data from crystal field spectra. In: Kieffer SW, Navrotsky A (eds) Reviews in Mineralogy, v. 14: Microscopic to Macroscopic, pp. 277–316, Mineralogical Society of America, Washington D.C.
Burns RG (1993) Minerogical applications of crystal field theory, second edition. Cambridge University Press
Fei Y, Mao HK, Mysen BO (1991) Experimental determination of element partitioning and calculation of phase relations in the MgO-FeO-SiO2 system at high pressure and high temperature. J Geophys Res 96:2157–2169
Fei Y, Virgo D, Mysen BO, Wang Y, Mao HK (1993) Temperature dependent electron delocalization in (Mg, Fe)SiO3-perovskite. Amer Mineral (submitted for publication)
Goto T, Ahrens TJ, Rossman GR, Syono Y (1980) Absorption spectrum of shock-compressed Fe2+-bearing MgO and radiative conductivity of the lower mantle. Phys Earth Planet Inter 22:277–288
Griffith JS (1961) Theory of transition metal ions. Cambridge Univ Press, London
Heinz DL, Jeanloz R (1987) Measurement of the melting curve of Mg0.9 Fe0.1 SiO3 at lower mantle conditions and its geophysical implications. J Geophys Res 92:11437–11444
Horiuchi H, Ito E, Weidner DJ (1987) Perovskite-type MgSiO3: Single crystal X-ray diffraction study. Amer Mineral 72:357–360
Huggins FE (1975) The 3d levels of ferrous iron in silicate garnets. Amer Mineral 60:316–319
Ito E, Takahashi E, Matsui Y (1984) The mineralogy and chemistry of lower mantle: an implication of the ultrahigh-pressure phase relations in the system MgO-FeO-SiO2. Earth Planet Sci Lett 67:238–248
Jackson WE, Knittle E, Brown GE Jr, Jeanloz R (1987) Partitioning of Fe within high-pressure silicate perovskite: Evidence for unusual geochemistry in the lower mantle. Geophys Res Lett 14:224–226
Jeanloz R, O'Neill B, Pasternak MP, Taylor RD, Bohlen SR (1991) Mössbauer spectroscopy of Mg0.9 Fe0.1 SiO3 perovskite (abstract). EOS Trans AGU 72(44) Fall Meeting Suppl: 464
Kudoh Y, Prewitt CT, Finger LW, Darovskikh A, Ito E (1990) Effect of iron on the crystal structure of (Mg, Fe)SiO3 perovskite. Geophys Res Lett 17:1481–1484
Marezio M, Remeika JP, Dernier PD (1970) The crystal chemistry of the rare earth orthoferrites. Acta Crystal B26:2008–2022
McCammon CA, Rubie DC, Ross II CR, Seifert F, O'Neill HStC (1992) Mössbauer spectra of 57Fe0.05 Mg0.95 SiO3 perovskite at 80 K and 298 K. Amer Mineral 77:894–897
Parise JB, Wang Y, Yeganeh-Haeri A, Cox DE, Fei Y (1990) Crystal structure and thermal expansion of (Mg, Fe)SiO3 perovskite. Geophys Res Lett 17:2089–2092
Ross HL, Hazen RM (1990) High-pressure crystal chemistry of MgSiO3 perovskite. Phys Chem Minerals 17:228–237
Sasaki S, Takeuchi Y, Fujino K, Akimoto S (1982) Electron-density distributions of three orthopyroxenes, Mg2Si2O6, Co2Si2O6, and Fe2Si2O6. Z Kristallogr 158:279–297
Shen G, Zhao M (1984) Analysis of the spectrum of Fe2+ in Fepyrope garnets. Phys Rev 30:3691–3703
Sherman DM (1991) The high pressure electronic structure of magnesiowüstite (Mg, Fe)O: Applications to the physics and chemistry of the lower mantle. J Geophys Res 96:14299–14312
Smyth JR, Bish DL (1988) Crystal structures and cation sites of the rock-forming minerals. pp 332, Allen & Unwin, Boston
Wang Y, Guyot F, Liebermann RC (1992) Electron microscopy of (Mg, Fe)SiO3 perovskite: Evidence for structural phase transitions and implications for the lower mantle. J Geophys Res 97:12327–12347
White WB, Moore RK (1972) Interpretation of the spin-allowed bands of Fe2+ in silicate garnets. Amer Mineral 57:1692–1710
Yagi T, Mao HK, Bell PM (1978) Structure and crystal chemistry of perovskite-type MgSiO3. Phys Chem Minerals 3:97–110
Zhao MG, Du ML (1983) Two center transitions in the antiferromagnetic salt FeCO3. Phys Rev B28:6481–6484
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shen, G., Fei, Y., Hålenius, U. et al. Optical Absorption Spectra of (Mg, Fe)SiO3 Silicate Perovskites. Phys Chem Minerals 20, 478–482 (1994). https://doi.org/10.1007/BF00203217
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203217