Abstract
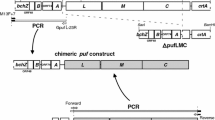
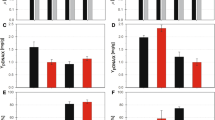
The gene (vgb) encoding the hemoglobin (VtHb) of Vitreoscilla sp. was cloned into a broad-host-range vector and stably transformed into Pseudomonas putida, Pseudomonas aeruginosa, and Xanthomonas maltophilia. vgb was stably maintained and expressed in functional form in all three species. When growth of the P. aeruginosa and X. maltophilia transformants in Luria-Bertani medium was compared with that of each corresponding untransformed strain, the VtHb-producing strains reached slightly higher maximum viable cell numbers, had significantly increased viability after extebded times in culture, and, like E. coli that produces VtHb, had significantly lower respiration rates. The VtHb-producing strain of P. putida also reached a slightly higher maximum viable cell number than its corresponding untransformed strain, but was significantly less viable after extended times in culture and, unlike the case in E. coli, had a generally higher respiration rate than the untransformed strain. When growth was monitored by absorbance, the results were similar to those obtained with viable cell counts.
Similar content being viewed by others
References
Bagdasarian M, Timmis KN (1981) Host: Vector system for gene cloning in Pseudomonas. In: Hofschneider PH, Goebel W (eds) Current topics in microbiology and immunology. Springer, Berlin, Heidelberg New York pp 47–67
Cheah UE, Weigand W, Stark BC (1987) Effects of recombinant plasmid size on cellular processes in Escherichia coli. Plasmid 18:127–134
Costerton JWF, Murray RGE, Robinow CF (1961) Observations on the mobility and structure of Vitreoscilla. Can J Microbiol 7:329–339
DeMondena JA, Gutiérrez S, Velasco J, Fernández F, Fachini RA, Galazzo JL, Hughes DE, Martin JF (1993) The production of cephalosporin C by Acremonium chrysogenum is improved by the intracellular expression of a bacterial hemoglobin. Biotechnology 11:926–929
Dikshit KL, Webster DA (1988) Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 70:377–386
Dikshit KL, Dikshit RP, Webster DA (1990) Study of Vitreoscilla globin (vgb) gene expression and promoter activity in E. coli through transcriptional fusion. Nucleic Acids Res 18:4149–4155
Georgiou C, Webster DA (1987) Purification and partial characterization of the membrane-bound cytochrome o (561, 564) from Vitreoscilla. Biochemistry 26:6521–6526
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197
Khosla C, Bailey JE (1988a) The Vitreoscilla hemoglobin gene: molecular cloning, nucleotide sequence, and genetic expression in Escherichia coli. Mol Gen Genet 214:158–161
Khosla C, Bailey JE (1988b) Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinant Escherichia coli. Nature 331:633–635
Khosravi M (1991) Use of genetic engineering to optimize protein production in recombinant Escherichia coli. PhD thesis, Illinois Institute of Technology, Chicago
Khosravi M, Webster DA, Stark BC (1990) Presence of the bacterial hemoglobin gene improves α-amylase production of a recombinant E. coli strain. Plasmid 24:190–194
Kranz RG, Gennis RB (1983) Immunological characterization of the cytochrome o terminal oxidase from Escherichia coli. J Biol Chem 258:10614–10621
Kushner SR (1978) An improved method for transformation of Escherichia coli with ColE-derived plasmids. In: Boyer HW, Nicosia S (eds) Genetic engineering: proceeding of the international symposium of genetic engineering, Milan, March 1978. Elsevier, Amsterdam, p 17
Liu CY, Webster DA (1974) Spectral characteristics and interconversions of the reduced, oxidized, and oxygenated forms of purified cytochrome o. J Biol Chem 249:4261–4266
Liu S-C (1994) Expanded study of the Vitreoscilla hemoglobin gene: DNA sequencing, cloning and expression in Pseudomonas. PhD thesis, Illinpis Institute of Technology, Chicago
Liu S-C, Ogretman B, Chuang Y-Y, Stark BC (1992) Selection and characterization of α-amylase-overproducing recombinant Escherichia coli containing the bacterial hemoglobin gene. Appl Microbiol Biotechnol 38:239–242
Liu S-C, Liu Y, Webster DA, Stark BC (1994) Sequence of the region downstream of the Vitreoscilla hemoglobin gene: vgb is not part of a multigene operon. Appl Microbiol Biotechnol 42:304–308
Mercer AA, Loutit JS (1979) Transformation and transfection of Pseudomonas aeruginosa: effects of metal ions. J Bacteriol 140:37–42
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, p 433
Potts M,. Angeloni SV, Ebel RE, Bassam D (1992) Myoglobin in a cyanobacterium. Science 256:1690–1692
Pringsheim EG (1951) The Vitreoscillaceae: a family of colorless, gliding, filamentous organisms. J Gen Microbiol 5:124–149
Rice CW, Hempfling WP (1978) Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol 134:115–124
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 1.25–1.29
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a dimeric bacterial hemoglobin from Vitreoscilla. Nature 322:481–483
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains. 33:103–119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, SC., Webster, D.A. & Stark, B.C. Cloning and expression of the Vitreoscilla hemoglobin gene in pseudomonads: effects on cell growth. Appl Microbiol Biotechnol 44, 419–424 (1995). https://doi.org/10.1007/BF00169938
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169938