Abstract
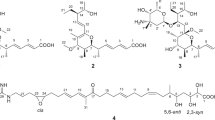
Actinoplanic acids A and B are macrocyclic polycarboxylic acids that are potent reversible inhibitors of farnesyl-protein transferase. Actinoplanic acids A and B were isolated from Actinoplanes sp. MA 7066 while actinoplanic acid B was isolated from both MA 7066 and Streptomyces sp. MA 7099. Actinoplanic acids A and B are competitive with respect to farnesyl diphosphate and are selective inhibitors of farnesyl-protein transferase because they do not inhibit geranylgeranyl-protein transferase type 1 or squalene synthase. MA 7066 is believed to be a novel species of actinomycetes while MA 7099 is believed to be a novel strain of Streptomyces violaceusniger on the basis of morphological, biochemical and chemotaxonomic characteristics as well as its production of actinoplanic acids.
Similar content being viewed by others
References
Barbacid M (1987) Ras genes. Annu Rev Biochem 56:779–827
Clarke S (1992) Protein isoprenylation and methylation at carboxyl-termini cysteine residues. Annu Rev Biochem 61:355–386
Der CJ, Cox AD (1991) Isoprenoid modification and plasma membrane association: critical factors for ras oncogenicity. Cancer Cells 3:331–340
Dufresne C, Wilson KE, Singh SB, Zink, DL, Bergstrom JD, Rew D, Polishook JD, Meinz MS, Huang L, Silverman KC, Lingham RB, Mojena M, Cascales C, Peláez F, Gibbs JB (1993) Zaragozic acids D and D2: potent inhibitors of squalene synthase and of Ras farnesyl protein transferase. J Nat Prod 56:1923–1929
Garcia AM, Rowell C, Ackermann K, Kowalczyk JJ, Lewis MD (1993) Peptidomimetic inhibitors of Ras farnesylation and function in whole cells. J Biol Chem 268:18415–18418
Gibbs JB (1991) Ras C-terminal processing enzymes — new drug targets? Cell 65:1–4
Gibbs JB (1992) Pharmacological probes of Ras function. Semin Cancer Biol 3:383–390
Gibbs JB, Schaber MD, Schofield T L, Scolnick EM, Sigal IS (1989) Xenopus oocyte germinal-vesicle breakdown induced by [Val12]Ras is inhibited by a cytosol-localized Ras mutant. Proc Natl Acad Sci USA 86:6630–6634
Gibbs JB, Pompliano DL, Mosser SD, Rands E, Lingham RB, Singh SB, Scolnick EM, Kohl NE, Oliff A (1993) Selective inhibition of farnesyl-protein transferase blocks Ras processing in vivo. J Biol Chem 268:7617–7620
Gibbs JB, Oliff A, Kohl NE (1994) Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell 77:175–178
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Hara M, Akasaka K, Akinaga S, Okabe M, Nakano H, Gomez R, Wood D, Uh M, Tamanoi F (1993) Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci USA 90:2281–2285
James GL, Goldstein JL, Brown MS, Rawson TE, Somers TC, McDowell RS, Crowley CW, Lucas BK, Levinson AD, Marsters JC (1993) Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science 260:1937–1942
Kohl NE, Mosser SD, deSolms SJ, Giuliani EA, Pompliano DL, Graham SL, Smith RL, Scolnick EM, Oliff A, Gibbs JB (1993) Selective inhibition of Ras-dependent transformation by a farnesyltransferase inhibitor. Science 260:1934–1937
Kohl NE, Wilson FR, Mosser SD, Giuliani E, DeSolms SJ, Conner MW, Anthomy NJ, Holtz WJ, Gomez RP, Lee TJ, Smith RL, Graham SL, Hartman GD, Gibbs JB, Oliff A (1994) Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci 91 USA:9141–9145
Lechevalier H, Lechevalier MP (1980) In: Dietz A, Thayer DW (eds) The chemotaxonomy of actinomycetes in actinomycete taxonomy. Society for Industrial Microbiology, Arlington, Va
Lingham RB, Silverman KC, Bills GF, Cascales C, Sánchez M, Jenkins RG, Gartner SE, Martin I, Diez MT, Peláez F, Mochales S, Kong YL, Burg RW, Meinz MS, Huang L, Nallin-Omstead M, Mosser SD, Schaber MD, Omer CA, Pompliano DL, Gibbs JB, Singh SB (1993) Chaetomella acutiseta produces chaetomellic acids A and B which are reversible inhibitors of farnesyl-protein transferase. Appl Microbiol Biotechnol 40:370–374
Liu WC, Barbacid M, Bulgar M, Clark LM, Crosswell AR, Dean L, Doyle TW, Fernandes PB, Huang S, Manne V, Pirnik DM, Wells JS, Meyers E (1992) 10'-Desmethoxystreptonigrin, a novel analog of streptonigrin. J Antibiot (Tokyo) 45:454–457
Locci R (1989) Section 29 streptomycetes and related genera. In: Williams ST, Sharp ME, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 4. Williams and Wilkins, Baltimore, MD, USA. pp 2451–2492
Miller I, Berger T (1985) Hewlett-Packard application note. Hewlett-Packard Co., Palo Alto, Calif, pp 228–241
Moores SL, Schaber MD, Mosser SD, Rands E, O'Hara MB, Garsky VM, Marshall S, Pompliano DL, Gibbs JB (1991) Sequence dependence of protein isoprenylation. J Biol Chem 266:14603–14610
Nonomura H (1974) Key for classification and identification of 458 species of the Streptomycetes included in ISP. J Ferment Technol 52:78–92
Nonomura H, Takagi S (1977) Distribution of actinoplanetes in soils of Japan. J Ferment Technol 55:423–428
Omer CA, Kral AM, Diehl RE, Prendergast GC, Powers S, Allen CM, Gibbs JB, Kohl NE (1993) Characterization of recombinant human farnesyl-protein transferase: cloning, expression, farnesyl diphosphate binding and functional homology with yeast prenyl-protein transferases. Biochemistry 32:5167–5176
Omura S, Van Der Pyl D, Inokoshi J, Takahashi Y, Takeshima H (1993) Pepticinnamins, new farnesyl-protein transferase inhibitors produced by an actinomycete. J Antibiot (Tokyo) 46:222–228
Pridham TG, Tresner HD (1974) Family VII. Streptomycetaceae Waksman and Henrici 1943, 339. In: Buchanan RE, Gibbons NE (eds) Bergey's manual of determinative bacteriology, 8th edn. Williams and Wilkins, Baltimore, MD, USA. pp 747–845
Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS (1990) Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 62:81–88
Reiss Y, Stradley SJ, Gierasch LM, Brown MS, Goldstein JL (1991a) Sequence requirements for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci USA 88:732–736
Reiss Y, Seabra MC, Armstrong SA, Slaughter CA, Goldstein JL, Brown MS (1991b) Nonidentical subunits of p21H-ras farnesyl-traserase. J Biol Chem 266:10672–10677
Rodenhuis S (1992) Ras and human tumors. Semin Cancer Biol 3:2451–2457
Shirling EB, Gottleib D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Shirling EB, Gottleib D (1968) Cooperative description of type cultures of Streptomyces. II Species descriptions from first study. Int J Syst Bacteriol 18:69–189
Singh SB, Zink DL, Liesch JM, Goetz MA, Jenking RG, Nallin-Omstead M, Silverman KC, Bills GF, Mosley RT, Gibbs JB, Albers-Schonberg G, Lingham RB (1993) Isolation and structures of chaetomellic acids A and B from Chaetomella acutiseta: farnesyl pyrophosphate mimics inhibitors of Ras farnesyl-protein transferase. Tetrahedron 49:5917–5926
Singh SB, Jones ET, Goetz MA, Bills GF, Nallin-Omstead M, Jenkins RG, Lingham RB, Silverman KC, Gibbs JB (1994a) Fusidienol: a novel inhibitor of Ras farnesyl-protein transferase from Fusidium griseum. Tetrahedron Lett 27:4693–4696
Singh SB, Liesch JM, Lingham RB, Goetz MA, Gibbs JB (1994b) Actinoplanic acid A: a macrocyclic polycarboxylic acid which is a potent inhibitor of Ras farnesyl-protein transferase. J Am Chem Soc 116:11606–11607
Tamanoi F (1993) Inhibitors of Ras farnesyltransferase. Trends Biochem Sci 18:349–353
US Department of Commerce, National Bureau of Standards (1985) NBS Circular 553 [Suppl]. National Bureau of Standards, Washington
Van Der Pyl D, Inokoshi J, Shioma K, Yang H, Takeshima H, Omura S (1992) inhibition of farnesyl-protein transferase by gliotoxin and acetylgliotoxin. J Antibiot (Tokyo) 45:1802–1805
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Silverman, K.C., Cascales, C., Genilloud, O. et al. Actinoplanic acids A and B as novel inhibitors of farnesyl-protein transferase. Appl Microbiol Biotechnol 43, 610–616 (1995). https://doi.org/10.1007/BF00164762
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164762