Abstract
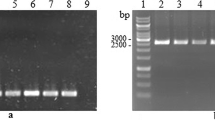
Conditions suitable for the production and regeneration of Pleurotus ostreatus protoplasts from dikaryotic mycelia were examined. Three commercially available muralytic enzymes, including Sigma lysing enzyme, Novozym 234 and Novozym 234 LP, were used for production of protoplasts. Over 2 × 107 protoplasts per gram fresh weight mycelia were obtained within 1.5 h by using each of these three enzymes. The colony regeneration rate was up to 13% on potato-dextrose-agar medium containing 0.8 m mannitol. Genetic transformation was based on positive selection for resistance to hygromycin B (HmB) using the plasmid vector pAN7-1 and accomplished by either electroporation or a polyethylene glycol (PEG)-divalent cation method. P. ostreatus strains used in this study have innate sensitivity to HmB at a critical inhibitory concentration of between 40–50 μg/ml. Selection for HmB resistance of this fungus, indicative of transformation, resulted in 3–48 HmB-resistant colonies per microgram of pAN7-1 per 107 viable protoplasts. No significant differences were apparent when either transformation protocol or either P. ostreatus strain was used. The best electrical condition found for the electrotransformation of P. ostreatus is at a field strength of 2.6–2.8 kV/cm with a capacitance of 25μF and a parallel resistance of 800 ohms, corresponding to a time constant range of 10–14 ms.
Similar content being viewed by others
References
Alic M, Kornegay JR, Pribnow D, Gold MH (1989) Transformation by complementation of an adenine auxotroph of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 55:406–411
Alic M, Mayfield MB, Akileswaran L, Gold MH (1991) Homologous transformation of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Curr Genet 19:491–494
Banks GR, Taylor SY (1988) Cloning of the PYR 3 gene of Ustilago maydis and its use in DNA transformation. Mol Cell Biol 8:5417–5424
Barrett V, Lemke PA, Dixon RK (1989) Protoplast formation from selected species of ectomycorrhizal fungi. Appl Microbiol Biotechnol 30:381–387
Barrett V, Dixon KD, Lemke PA (1990) Genetic transformation of a mycorrhizal fungus. Appl Microbiol Biotechnol 33:313–316
Bej AK, Perlin MH (1989) A high efficiency transformation system for the basidiomycete Ustilago violacea employing hygromycin resistance and lithium-acetate treatment. Gene 80:171–176
Bej AK, Perlin MH (1991) Acquisition of mitochondrial DNA by a transformation vector for Ustilago violacea. Gene 98:135–140
Binninger DM, Skrzynia C, Pukkila PJ, Casselton LA (1987) DNA-mediated transformation of the basidiomycete Coprinus cinereus EMBO J 6:835–840
Binninger DM, Chevanton LL, Skrzynia C, Shubkin CD, Pukkila PJ (1991) Targeted transformation in Coprinus cinereus. Mol Gen Genet 227:245–251
Burrows DM, Elliott TT, Casselton LA (1990) DNA-mediated transformation of the secondarily homothallic basidiomycete Coprinus bilanatus. Curr Genet 17:175–177
Byun MO, Yoo YB, Go SJ, You CH, Cha DY, Park YH (1989) Transformation of the β-isopropylmalate dehydrogenase gene of Flammulina velutipes into Pleurotus florida. Korean J Mycol 17:27–30
Chakraborty BN, Kapoor M (1990) Transformation of filamentous fungi by electroporation. Nucleic Acids Res 18:6737
Challen MP, Rao BG, Elliott TJ (1991) Transformation strategies for Agaricus. In: Griensven LJLD van (ed) Genetics and breeding of Agaricus. Pudoc, Wageningen, The Netherlands, pp 129–134
Chang ST, Miles PG (1989) Edible mushrooms and their cultivation. CRC Press, Boca Raton, Fla.
Chang ST, Miles PH (1991) World production of edible mushrooms. Mushroom J 504:15–18
Fincham JRS (1989) Transformation in fungi. Microbiol Rev 53:148–170
Finkelstein DB (1992) Transformation. In: Finkelstein DB, Ball C (eds) Biotechnology of filamentous fungi; technology and products. Butterworth-Heinemann, Stoneham, pp 113–156
Goldman GH, Montagu M van, Herrera-Estrella A (1990) Transformation of Trichoderma harzianum by high-voltage electric pulse. Curr Genet 17:169–174
Hargreaves JA, Turner G (1989) Isolation of the acetyl-CoA synthase gene from the corn smut pathogen, Ustilago maydis. J Gen Microbiol 135:2675–2678
Hondel CAMJJ van den, Punt PJ (1991) Gene transfer systems and vector development for filamentous fungi. In: Peberdy JF, Caten CE, Ogden JE, Bennett JW (eds) Applied molecular genetics of fungi. Cambridge University, Cambridge, pp 1–28
Horgen PA, Anderson JB (1992) Edible mushrooms. In: Finkelstein DB, Ball C (eds) Biotechnology of filamentous fungi; technology and products. Butterworth-Heinemann, Stoneham, pp 447–462
Iijima Y, Yanagi SO (1986) A method for the high yield preparation of and high frequency regeneration of basidiomycete, Pleurotus ostreatus (“Hiratake”), protoplasts using sulfite pulp waste components. Agric Biol Chem 50:1855–1861
Lau WC, Dhillon, EKS, Chang ST (1985) Isolation and reversion of Protoplasts of Pleurotus sajor-caju. MIRCEN J 1:191–194
Magae Y, Kakimoto Y, Kashiwagi Y, Sasaki T (1985) Fruiting body formation from regenerated mycelium of Pleurotus ostreatus protoplasts. Appl Environ Microbiol 49:441–442
Marmeisse R, Gay G, Debaud JC, Casselton LA (1992) Genetic transformation of the symbiotic basidiomycetes Hebeloma cylindrosporum. Curr Genet 22:41–45
Munroz-Rivas A, Specht CA, Ullrich RC (1986) Transformation of the basidiomycete, Schizophyllum commune. Mol Gen Genet 205:103–106
Peberdy JF (1989) Fungi without coats — protoplasts as tools for mycological research. Mycol Res 93:1–20
Peng M, Singh NK, Lemke PA (1992) Recovery of recombinant plasmids from Pleurotus ostreatus transformants. Curr Genet 22:53–59
Punt PJ, Oliver RP, Dingemansi MA Pouwels PH, Hondel CAMJJ van den (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124
Richey MG, Marek ET, Schardl CL, Smith DA (1989) Transformation of filamentous fungi with plasmid DNA by electroporation. Phytopathology 79:844–847
Royer JC, Horgen PA (1991) Towards a transformation system for Agaricus bisporus. In: Griensven LJLD van (ed) Genetics and breeding of Agaricus. Pudoc, Wageningen, The Netherlands, pp 135–139
Royse DJ (1992) Recycling of spent shiitake substrate for production of the oyster mushroom, Pleurotus sajor-caju. Appl Microbiol Biotechnol 38:179–182
Specht CA, Munoz-Rivas A, Novotny CP, Ullrich RC (1988) Transformation of Schizophyllum commune: analysis of parameters for improving transformation frequencies. Exp Mycol 12:257–366
Specht CA, Stankis MM, Giasson L, Novotny CP, Ullrich RC (1992) Functional analysis of the homeodomain-related proteins of the Aα locus of Schizophyllum commune. Proc Natl Acad Sci USA 89:7174–7178
Toyomasu T, Matsumoto T, Mori K (1986) Interspecific protoplast fusion between Pleurotus ostreatus and Pleurotus salmoneo-stramineus. Agric Biol Chem 50:223–225
Tsukuda T, Carleton S, Fotheringham S, Holloman WK (1988) Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol 8:3703–3709
Wakabayashi S, Magae Y, Kashiwagi Y, Sasaki T (1985) Formation of giant protoplasts from protoplasts of Pleurotus cornucopiae by the cell wall lytic enzyme. Appl Microbiol Biotechnol 21:328–330
Wang J, Holden DH, Leong SA (1988) Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci USA 85:865–869
Ward M, Kodama KH, Wilson LJ (1989) Transformation of Aspergillus awamori and A. niger by electroporation. Exp Mycol 13:289–293
Yamada O, Magae Y, Kashiwagi Y, Kakimoto Y, Sasaki T (1983) Preparation and regeneration of mycelial protoplasts of Collybia veltipes and Pleurotus ostreatus, Eur J Appl Microbiol Biotechnol 17:298–300
Yelton M, Hamer JF, Timberlake WE (1984) Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci USA 81:1470–1474
Author information
Authors and Affiliations
Additional information
Correspondence to: P. A. Lemke
Rights and permissions
About this article
Cite this article
Peng, M., Lemke, P.A. & Shaw, J.J. Improved conditions for protoplast formation and transformation of Pleurotus ostreatus . Appl Microbiol Biotechnol 40, 101–106 (1993). https://doi.org/10.1007/BF00170436
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170436