Summary
The absorbable deoxynojirimycin derivative emiglitate (BAY o 1248) is a potent competitive inhibitor of small intestinal α-glucosidases in man.
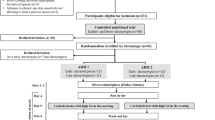
In two similar randomized, placebo-controlled, double blind investigations, the efficacy, duration of action and tolerability of single doses of 10, 20 and 40 mg emiglitate have been assessed in healthy male volunteers after repeated sucrose or maize-starch loads at 08.00, 12.00 and 17.00 h.
Even at the lowest dose used, emiglitate almost abolished the glycaemic (−88%) and hormonal responses after the first sucrose meal, simultaneously evoking significant hydrogen evolution (mean peak H2-concentration >100 ppm), which was not related to the dose, and which induced unacceptable symptoms of carbohydrate malabsorption, i.e. at the dosages tested, the inhibition of glycaemic and hormonal responses was at the expense of intolerable gastrointestinal adverse effects.
Flattening of postprandial responses of blood glucose, serum insulin and gastric inhibitory polypeptide was still apparent after a second sucrose load 4 h later, demonstrating long-lasting inhibition of α-glucosidase activity.
After starch, the dose dependency of inhibition emerged more clearly than after sucrose, i.e. the reduction was less pronounced. However, emiglitate led to significant reduction of the glycaemic and hormonal rises after both the first and second starch meals. Symptoms of carbohydrate malabsorption were absent after 10 mg and were negligible with 20 mg or 40 mg emiglitate. Breath hydrogen concentration increased gradually, indicating slight but significant carbohydrate malabsorption after the highest dose of the α-glucosidase inhibitor.
The results show that a single morning dose of 20–40 mg emiglitate might be useful in the control of postprandial hyperglycaemia after breakfast and lunch. This dose of the inhibitor was effective after either both 50 g starch or 50 g sucrose as the substrate, but was only tolerable after the starch meal.
Similar content being viewed by others
References
Jones B, Brown BE, Loran JS, Edgerton D, Kennedy JF, Stead JA, Silk DBA (1983) Glucose absorption from starch hydrolysates in the human jejunum Gut 24: 1152–1160
McMichael HB, Webb J, Dawson AM (1967) The absorption of maltose and lactose in man. Clin Sci 33: 135–145
Puls W, Krause HP, Müller L, Schutt H, Sitt G, Thomas G (1984) Inhibitors of the rate of carbohydrate and lipid absorption by the intestine. Intern J Obesity 8 (suppl 1): 181–190
Creutzfeldt W (ed) (1982) Proceedings/First international symposium on acarbose. Exerpta Medica, Amsterdam
Dimitriadis G, Tessari P, Go V, Gerich J (1985) α-Glucosidase inhibition improves postprandial hyperglycemia and decreases insulin requirements in insulin-dependent diabetes mellitus. Metabolism 34: 261–265
Gerard J, Luyckx A, Lefebvre P (1981) Improvement of metabolic control in insulin dependent diabetics treated with the α-glucosidase inhibitor acarbose for two months. Diabetologia 21: 446–451
Sachse G, Willms B (1979) Effect of the α-glucosidase-inhibitor BAY g 5421 on blood glucose control of sulfonylurea-treated diabetics and insulin-treated diabetics. Diabetologia 17: 287–290
Pütter J (1980) Studies on the pharmacokinetics of Acarbose in humans. In: U Brodbeck (ed): Enzyme Inhibitors. Verlag Chemie, Weinheim, p 139–151
Ruppin H, Hagel J, Feuerbach W, Schutt H, Pichl J, Hillebrand I, Bloom S, Domschke W (1988) Fate and effects of the α-glucosidase inhibitor acarbose in humans. Gastroenterology 95: 93–99
Rämsch K-D, Wetzelsberger N, Pütter J, Maul W (1985) Pharmacokinetics and meteabolism of the desoxynojirimycin derivatives BAY m 1099 and BAY o 1248. Diabetes Res Clin Pract 1 [Suppl 1]: 459 (A)
Bischoff H, Puls W, Krause HP, Schutt H, Thomas G (1985) Pharmacological properties of the novel glucosidase inhibitors BAY m 1099 (miglitol) and BAY o 1248. Diabetes Res Clin Pract 1 [Suppl 1]: 53
Lembcke B, Fölsch UR, Creutzfeldt W (1985) Effect of 1-desoxynojirimycin derivatives on small intestinal disaccharidase activities and on active transport in vitro. Digestion 31: 120–127
Caspary WF, Graf S (1979) Inhibition of human intestinal α-glucosidehydrolases by a new complex oligosaccharide. Res Exp Med (Berlin) 175: 1–6
Taylor RH, Barker HM, Bowey EA, Canfield JE (1986) Regulation of the absorption of dietary carbohydrate in man by new glycosidase inhibitors. Gut 27: 1471–1478
Thompson DG, Wingate DL, Thomas M, Harrison D (1982) Gastric emptying as a determinant of the oral glucose tolerance test. Gastroenterology 82: 51–55
Ebert R, Illmer K, Creutzfeldt W (1979) Release of gastric inhibitory polypeptide (GIP) by intraduodenal acidification in rats and humans and abolishment of the incretin effect of acid by GIP-antiserum in rats. Gastroenterology 76: 515–523
Metz GL, Gassull MA, Leeds AR, Blendis LM, Jenkins DJA (1976) A simple method of measuring breath hydrogen in carbohydrate malabsorption by end-expiratory sampling. Clin Sci Mol Med 50: 237–240
Lembcke B, Caspary WF (1982) Wasserstoff (H2)-Exhalationstests in der gastroenterologischen Funktionsdiagnostik — Apparative und methodische Aspekte. Lab Med 6: 186–193
Schmidt DD, Frommer W, Müller L, Truscheit E (1979) Glucosidase-Inhibitoren aus Bazillen. Naturwissenschaften 66: 584–585
Lembcke B, Löser C, Fölsch UR, Wöhler J, Creutzfeldt W (1987) Adaptive responses to pharmacological inhibition of small intestinal α-glucosidases in the rat. Gut 28 [Suppl 1]: 181–187
Cauderay M, Tappy L, Temler E, Jequier E, Hillebrand I, Felber J-P (1985) Effect of α-glycohydrolase inhibitors (BAY m 1099 and BAY o 1248) on sucrose metabolism in normal men. Metabolism 35: 472–477
Taylor RH, Jenkins DJA, Barker H, Fielden H, Goff DV, Misiewicz JJ, Lee DA, Allen HB, MacDonald G, Wallrabe H (1982) Effect of acarbose on the 24-hour blood glucose profile and pattern of carbohydrate absorption. Diabetes Care 5: 92–96
Hillebrand I, Boehme K, Graefe KH, Wehling K (1986) The effect of new α-glucosidase inhibitors (BAY m 1099 and BAY o 1248) on meal-stimulated increases in glucose and insulin levels in man. Klin Wochenschr 64: 393–396
Dimitriadis G, Hatziagelaki E, Ladas S, Linos A, Hillebrand I, Raptis S (1988) Effects of prolonged administration of two new α-glucosidase inhibitors on blood glucose control, insulin requirements and breath hydrogen excretion in patients with insulin-dependent diabetes mellitus. Eur J Clin Invest 18: 33–38
Uttenthal LO, Ukponmwan OO, Ghiglione M, Bloom SR (1987) Acute and short term effects of intestinal alpha-glucosidase inhibition on gut hormone responses in man. Dig Dis Sci 32: 139–144
Creutzfeldt W (1979) The incretin concept today. Diabetologia 16: 75–85
Lembcke B, Kirchhoff S, Fölsch UR, Creutzfeldt W (1985) Wirkung von α-Amylase- und α-Glukosidase-Inhibitoren auf jejunale Disaccharidasen des Menschen in vitro. Z Gastroenterol 23: 472
Lembcke B (1987) Untersuchungen zur medikamentösen Beeinflussung der intestinalen Kohlenhydratassimilation. Ein Beitrag zum Therapieprinzip Resorptionsverzögerung. Habilitations-schrift, Medizinische Fakultät, Göttingen
Mayer KH, Stamler J, Dyer A, Feinkel N, Stamler R, Berkson DM, Farber B (1976) Epidemiologic findings on the relationship of time of day and time since last meal to glucose tolerance. Diabetes 25: 936–943
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lembcke, B., Fölsch, U.R., Gatzemeier, W. et al. Inhibition of sucrose- and starch-induced glycaemic and hormonal responses by the α-glucosidase inhibitor emiglitate (BAY o 1248) in healthy volunteers. Eur J Clin Pharmacol 41, 561–567 (1991). https://doi.org/10.1007/BF00314985
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00314985