Summary
The single-dose and steady state kinetics of morphine given as controlled-release tablets (30 mg every 12 h) and as a solution (15 mg every 6 h) have been compared in 11 cancer patients with chronic pain. The concentrations of morphine, morphine-3-glucuronide (M3G), and morphine-6-glucuronide (M6G) were analyzed by HPLC.
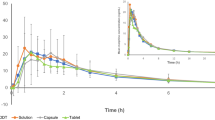
There were no significant differences between the tablets and solution in the mean steady state concentrations of morphine, M3G or M6G. The tmax was 3.3 h for the tablets compared to 1.1 h for the solution.
After giving the controlled-release tablets every 12 h there was a significantly higher fluctuation index of the morphine concentrations than after the solution. Urinary recovery at steady state was comparable between the two preparations, with averages of 57% and 47%, respectively.
Thus, no major differences were found in the pharmacokinetics of morphine and its glucuronidated metabolites after 30 mg morphine as controlled-release tablets every 12 h or 15 mg of morphine solution every 6 h, except for a significantly longer tmax and greater fluctuation in morphine concentrations after the controlled-release tablets.
Similar content being viewed by others
References
Iwamoto K, Klaassen C (1977) The first pass of morphine in rats. J Pharmacol Exp Ther 200: 236–244
Dahlström B, Paalzow L, Segre G, Ågren AJ (1978) Relation between morphine pharmacokinetics and analgesia. J Pharmacokinet Biopharm 5: 41–53
Säwe J, Kager L, Svensson Eng JO, Rane A (1985) Oral morphine in cancer patients: in vivo kinetics and in vitro hepatic glucuronidation. Br J Clin Pharmacol 19: 495–501
Säwe J (1985) Morphine and its 3- and 6-glucuronides during chronic oral administration in cancer patients. Adv Pain Res Ther 8: 45–55
Säwe J, Dahlström B, Paalzow L, Rane A (1981) Morphine kinetics in cancer patients. Clin Pharmacol Ther 30: 629–635
Twycross RG (1982) Morphine and diamorphine in the terminally ill patient. Acta Anaesth Scand 74 [Suppl]: 128–134
Hanks GW, Twycross RG, Bliss JM (1987) Controlled release morphine tablets: a double-blind trial in patients with advanced cancer. Anaesthesia 42: 840–844
Welsh J, Stuart JFB, Habeshaw T, Blackie R, Whitehill D, Setanoians A, Milsted RAV, Calman KC (1983) A comparative pharmacokinetic study of morphine sulphate solution and MST Continus® 30 mg tablets in conditions expected to allow steady state drug levels. In: Stuart JF (ed) Methods of morphine estimation in biological fluids and the concept of free morphine. Academic Press, London, pp 9–11
Knudsen J, Mortensen SM, Eikland B, Henriksen H (1985) Morfin-depot-tabletter og konventionelle morfintabletter vid cancersmerter. Ugeskr Laeger 148: 780–784
Savarese JJ, Goldenheim PD, Thomas GB, Kaiko RF (1986) Steady state pharmacokinetics of controlled release oral morphine sulphate in healthy subjects. Clin Pharmacokinet 11: 505–510
Poulain P, Hoskin PJ, Hanks GW, Omar OA, Walker VA, Johnston A, Turner P, Aherne GW (1988) Relative bioavailability of controlled release morphine tablets (MST Continus) in cancer patients. Br J Anaesth 61: 569–574
Svensson Eng J-O, Rane A, Säwe J, Sjöqvist F (1982) Determination of morphine, morphine-3-glucuronide and (tentatively) morphine-6-glucuronide in plasma and urine using ion-pair high-performance liquid chromatography. J Chromatogr 230: 427–432
Svensson Eng J-O (1986) Determination of morphine, morphine-6-glucuronide and normorphine in plasma and urine with high-performance liquid chromatography and electrochemical detection. J Chromatogr 375: 174–178
Lilliefors HW (1967) On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Ass 62: 399–402
Grizzle JE (1965) The two-period change-over design and its use in clinical trials. Biometrics 21: 467–480
Grizzle JE (1974) The two-period change-over design and its use in clinical trials. Biometrics 30: 727
Koch GG (1972) The use of non-parametric methods for the statistical analysis of the two-period change-over design. Biometrics 28: 577–584
Dahlström B, Tamsen A, Paalzow L, Hartvig P (1982) Patient-controlled analgesic therapy. Pharmacokinetics and analgesic plasma concentrations of morphine. Clin Pharmacokinet 7: 266–279
Säwe J (1984) Oral morphine and methadone in the treatment of cancer pain. Clinical pharmacokinetic studies. Doctoral thesis, Karolinska Institute, Stockholm, Sweden
Vater M, Smith G, Aherne GW, Aitkenhead AR (1984) Pharmacokinetics and analgesic effect of slow-release oral morphine sulphate in volunteers. Br J Anaesth 56: 821–827
Neumann PB, Henriksen H, Grosman N, Christensen CB (1982) Plasma morphine concentrations during chronic oral administration in patients with cancer pain. Pain 13: 247–252
Säwe J, Svensson JO, Rane A (1983) Morphine metabolism in cancer patients on increasing oral doses — no evidence for autoinduction or dose-dependence. Br J Clin Pharmacol 16: 85–93
Kager L, Ljungdahl I, Rane A, Säwe J (1979) Oral morphine treatment of pain in terminal cancer. Läkartidningen 76: 3411–3415
Aherne GW, Piall EM, Twycross RG (1979) Serum morphine concentration after oral administration of diamorphine hydrochloride and morphine sulphate. Br J Clin Pharmacol 8: 577–580
Brunk SF, Delle M (1974) Morphine metabolism in man. Clin Pharmacol Ther 16: 51–57
Shimomura K, Kamata O, Ueki S, Ida S, Oguri K, Yoshimura H, Tsukamoto H (1971) Analgesic effect of morphine glucuronides. Tohoku J Exp Med 105: 45–52
Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE (1987) Morphine-6-glucuronide, a potent mu agonist. Life Sci 41: 2845–2849
Osborne R, Joel S, Trew D, Slevin M (1988) Analgesic activity of morphine-6-glucuronide. Lancet I: 828
Osborne RJ, Joel SP, Slevin ML (1986) Morphine intoxication in renal failure: the role of morphine-6-glucuronide. Br Med J 292: 1548–1549
Hasselström J, Berg U, Löfgren A, Säwe J (1989) Long lasting respiratory depression induced by morphine-6-glucuronide? Br J Clin Pharmacol 27: 515–518
McQuay HJ, Carroll D, Faura CC, Gavaghan DJ, Hand CW, Moore RA (1990) Oral morphine in cancer pain: influences on morphine and metabolite concentration. Clin Pharmacol Ther 48: 236–244
Twycross RG (1974) Clinical experience with diamorphine in advanced malignant disease. Int J Clin Pharmacol Ther Toxicol 9: 184–198
Yeh SY (1973) Urinary excretion of morphine and its metabolites in morphine-dependent subjects. J Pharmacol Exp Ther 22: 1423–1430
Dahlström B, Paalzow L (1978) Pharmacokinetic interpretation of the enterohepatic recirculation and first-pass elimination of morphine in the rat. J Pharmacokinet Biopharm 6: 505–519
Shepard TA, Reuning RH, Aarons LJ (1985) Estimation of area under the curve for drugs subject to enterohepatic cycling. J Pharmacokinet Biopharm 13: 589–608
Karnofsky DA, Abelman WH, Craver LF, Burchenal JH (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1: 634–656
Osborne R, Joel S, Trew D, Slevin M (1990) Morphine and metabolite behavior after different routes of morphine administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther 47: 12–19
Weitz CJ, Faull KF, Goldstein A (1987) Synthesis of the skeleton of the morphine molecule by mammalian liver. Nature 330: 674–677
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasselström, J., Alexander, N., Bringel, C. et al. Single-dose and steady-state kinetics of morphine and its metabolites in cancer patients — a comparison of two oral formulations. Eur J Clin Pharmacol 40, 585–591 (1991). https://doi.org/10.1007/BF00279975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279975