Abstract
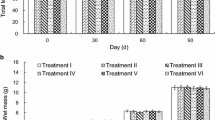
Ageing of abalone by counting rings laid down internally in the shell has presented problems of validation. Three methods of tetracycline marking were tested in Haliotis iris. Juvenile (<70 mm shell length) and adult (>115 mm) abalone were injected intramuscularly with tetracycline hydrochloride, injected with oxytetracycline, or immersed in tetracycline hydrochloride. After treatment, shells were cut sagitally and examined under ultraviolet light using a stereomicroscope. In treatments where juveniles were injected with tetracycline hydrochloride or oxytetracycline hydrochloride at dosages ranging from 20 to 600 mg per kg body weight, no fluorescent marks were visible from treatments≤80 mg/kg, but 83% of juveniles treated with greater dosages retained a visible mark. In treatments where juveniles were immersed in seawater solutions of tetracycline hydrochloride at five concentrations ranging from 200 to 1000 mg per litre of seawater and sampled at 5 h intervals for periods ranging from 5 to 40 h, all showed clear fluorescent markings. Shells of adult abalone injected with tetracycline hydrochloride at four dosages ranging from 200 to 800 mg/kg all showed clearly visible marks 18 d post-treatment. Abalone injected at dosages of 600 and 800 mg/kg exhibited tissue fluorescence around the injection site 2 wk after treatment. Adults immersed for 48 h at four concentrations ranging from 200 to 800 mg/l produced marks comparable to those of injected adults. Abalone were clearly stressed by some treatments. Only 50% of adults injected at 200 mg/kg were able to right themselves within 10 min, while all those injected at higher concentrations either were incapable of righting themselves after treatment or were extremely sluggish. All immersed adults quickly righted themselves. These results show that both injection and immersion are effective in marking abalone, but that immersion is less stressful to them.
Similar content being viewed by others
Literature cited
Beamish, R. J., MacFarlane, G. A. (1983). The forgotten requirements of age validation in fisheries biology. Trans. Am. Fish. Soc. 112: 735–743
Brown, C. A., Gruber, S. H. (1988). Age assessment of the lemon shark Negaprion brevirostris, using tetracycline validated vertebral central. Copcia 3: 747–753
Clark, G. R. (1974). Growth lines in invertebrate skeletons. A. Rev. Earth planet. Sciences 2: 77–99
Daiber, F. C. (1960). A technique for age determination in the skate, Raja enlanteria. Copeia 1960: 258–260
Ebert, T. A. (1985). The non-periodic nature of growth rings in echinoid spines. In: Keegan, B. F., O'Connor, B. D. S. (eds.) Echinodermata. Proceedings of the (5th International Echinoderm Conference). A. A. Balkema, Rotterdam, p. 261–267
Ebert, T. A. (1988). Calibration of natural growth lines in the ossicles of two sea urchins, Strongylocentrotus purpuratus & Echinoderm mathaei, using tetracycline. In: Burke, R. D., Mladenov, P. V., Lambert, P., Parsley, R. L. (eds.) Echinoderm biology. Proceedings of the 6th International Echinoderm Conference. A. A. Balkema, Rotterdam, p. 435–443
Forster, G. R. (1967). The growth of Haliotis tuberculata: results of tagging experiments in Gurnsey. J. mar. biol. Ass. U.K. 47: 287–300
Fritz, L. W., Haven, D. S. (1983). Hard clam Mercenaria mercenaria: shell growth patterns in Chesapeake Bay. Fish. Bull. U.S. 81: 697–708
Gage, J. D. (1991). Skeletal growth zones as age-markers in the sea urchin Pasammechinus miliaris. Mar. Biol. 110: 217–228
Hayashi, I. (1980). Structure and function of a shore population of ormer, Haliotis tuberculata J. mar. biol. Ass. U.K. 60: 431–437
Jackson, G. D. (1990). The use of tetracycline staining techniques to determine statolith growth ring periodicity in the tropical loliginid squids Loligo chinensis. Veliger 33: 389–393
Kim, J-W., Cheung, S.-C. (1985). On the growth of abalones, Subculus diversicolor diversicolor (Reeve) and S. diversicolor aquatilis (Reeve) in Cheju Island. Bull. mar. Resour. Res. Inst., Jeju natn. Univ. 9: 39–50
Kojima, H., Nakahisa, Y., Tanimoto, H. (1977). A study on the stock of Japanese black abalone, Haliotis discus discus in the Tokushima Prefecture. I. Growth of shells. Bull. Tokai reg. Fish. Res. Lab. 90: 25–37
Lipinski, M. (1986). Methods for the validation of squid age from statoliths. J. mar. biol. Ass. U.K. 66: 505–524
McFarlane, G. A., Beamish, R. J. (1987). Selection of dosages of oxytetracycline for age validation. Can. J. Fish. aquat. Sciences 44: 905–909
Milch, R. A., Rall, D. P., Tobie, J. E. (1957). Bone localization of the tetracyclines. J. natn. Cancer Inst. 19: 87–93
Murray, T. (1986). Paua shell and its growth. Shellfish. Newsl. 30: p. 10 [Suppl. to “Catch '86” (N.Z. Minist. Agric. Fish.)]
Murray, T., Akroyd, J. (1984). New Zealand paua fishery: an update and review of biological considerations to be reconciled with management goals. New Zealand Ministry of Agriculture and Fisheries, Fisheries Research Centre, Wellington (Internal Report No. 5)
Nakahara, H. (1961). Determination of growth rates of nacreous layer by the administration of tetracycline. Bull. natn. Pearl Res. Lab. 6: 607–614
Peterson, C. H., Duncan, P. B., Summerson H. C., Safrit, J. R. (1983). A mark — recapture test of periodicity of internal growth band deposition in shells of hard clams, Mercenaria mercenaria from a population along the Southeastern United States. Fish. Bull. U.S. 81: 765–779
Prince, J. D., Sellers, T. L., Ford, W. B., Talbot, S. R. (1988). A method for aging the abalone Haliotis rubra (Mollusca: Gastropoda). Aust. J. mar. Freshwat. Res. 39: 167–175
Sakai, S. (1960). On the formation of the annual ring on the shell of the abalone, Haliotis discus var. hannai Ino. Tohoku J. agric. Res. 239–244
Schiel, D. R. (1992). The paua (abalone) fishery of New Zealand. In: Shepherd, S. A., Tegner, M. J., Guzman del Proo S. (eds.) Abalone of the world: biology fisheries, and culture. Blackwell Scientific Oxford, p. 427–437
Schiel, D. R., Breen, P. A. (1991). Population structure, aging and fishing mortality of the New Zealand abalone Haliotis iris. Fish. Bull. U.S.: 89: 681–691
Schiel, D. R., Welden, B. C. (1987). Response to predators of cultured and wild red abalone, Haliotis rufescens, in laboratory experiments. Aquaculture, Amsterdam 60: 173–188
Summerfelt, R. C., Hall, G. E. (eds.) (1987). Age and growth of fish. Proceedings of the International Symposium on the Age and Growth of Fish, Des Moines, Iowa. Iowa State University Press, Ames, Iowa, USA
Tegner, M. J., Breen, P. A., Lennert, C. E. (1989). Population biology of red abalones, Haliotis rufescens in southern California and management of the red and pink, H. corrugata, abalone fisheries. Fish. Bull. U.S. 87: 313–339
Tegner, M. J., Butler, R. A. (1985). The survival and mortality of seeded and native red abalones, Haliotis rufescens, on the Palos Verdes Peninsula. Calif. Fish Game 71: 150–163
Tufts, N. R. (1967). Topical labelling of shellfish. Proc. natn. Shellfish. Ass. 57: 73–76
Weber, D. D., Ridgway, G. J. (1962). The deposition of tetracycline drugs in bones and scales of the fish and its possible use for marking. Progve Fish Cult. (October): 150–155
Weber, D. D., Ridgway, G. J. (1967). Marking Pacific salmon with tetracycline antibiotics. J. Fish. Res. Bd Can. 24: 849–865
Wilson, C. W., Beckman, D. W., Dean, J. M. (1987). Calcein as a fluorescent marker of otoliths of larval and juvenile fish. Trans. Am. Fish. Soc. 116: 668–670
Yamada, U. (1971). Vital staining of the hard tissues of carp Cyprinus carpio Linné with tetracycline-HCL, calcein and alizarin red S. Bull. Seikai Reg. Fish. Res. Lab. 41: 107–116
Author information
Authors and Affiliations
Additional information
Communicated by G. F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Pirker, J.G., Schiel, D.R. Tetracycline as a fluorescent shell-marker in the abalone Haliotis iris . Marine Biology 116, 81–86 (1993). https://doi.org/10.1007/BF00350734
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00350734