Abstract
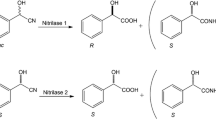
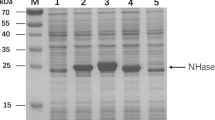
Bacteria were enriched from soil samples, using benzylcyanide, α-methyl-, α-ethyl- or α-methoxybenzyl-cyanide as the sole source of nitrogen. All isolated strains belonged to the genus Pseudomonas. Resting cells of the isolates hydrolysed O-acetylmandelonitrile to O-acetylmandelic acid, O-acetylmandelic acid amide and mandelic acid. From racemic O-acetylmandelonitrile all isolates preferentially formed R(−)-acetylmandelic acid ( = d-acetylmandelic acid). The enantioselective hydrolysis of O-acetylmandelonitrile could also be demonstrated in vitro. Crude extracts did not hydrolyse O-acetylmandelic acid amide indicating an enantioselective nitrilase rather than a nitrile hydratase/amidase system.
Similar content being viewed by others
References
Anschütz R, Böcker R (1909) Über die Einwirkung von Acetylman-delsäurechlorid auf Natriummalonsäureester und auf Natrium-cyanessigester. Liebigs Ann Chem 368: 53–75
Baker DP, Fewson CA (1989) Purification and characterization of d(−)-mandelate dehydrogenase from Rhodotorula graminis. J Gen Microbiol 135: 2035–2044
Bergstrom J, Bergstrom G (1989) Floral scents of Bartsia alpina (Scrophulariaceae). Chemical composition and variation between individual plants. Nord J Bot 9: 363–366
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Effenberger F, Ziegler T, Förster S (1987) Enzymkatalysierte Cyanhydrin-Synthese in organischen Lösungsmitteln. Angew Chem 99: 491–493
Effenberger F, Hörsch B, Förster S, Ziegler T (1990) Enzyme-catalyzed synthesis of (S)-cyanohydrins and subsequent hydrolysis to (S)-2-hydroxy-carboxylic acids. Tetrahedron Lett 31: 1249–1252
Effenberger F, Gutterer B, Ziegler T, Eckhardt E, Eichholz R (1991) Enantioselektive Veresterung racemischer Cyanhydrine und enantioselektive Hydrolyse oder Umesterung racemischer Cyanhydrinester mittels Lipasen. Liebigs Ann Chem 47–51
Endo T, Watanabe I (1989) Nitrile hydratase of Rhodococcus sp. N-774. Purification and amino acid sequences. FEBS Lett 32: 61–64
Gregersen T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotech 5: 123–127
Harper DB (1977a) Microbial metabolism of aromatic nitriles. Enzymology of C-N cleavage by Nocardia sp. (Rhodochrous group) NCIB 11216 Biochem J 165: 309–318
Harper DB (1977b) Fungal degradation of aromatic nitriles. Enzymology of C-N cleavage by Fusarium solani. Biochem J 167: 685–692
Hashimoto S, Kameoka H (1985) Sulfur- and nitrogen-containing neutral volatile components of Cruciferae. J Food Sc 50: 847–852
Hook RH, Robinson WG (1964) Ricine nitrilase. II. Purification and properties. J Biol Chem 53: 4263–4267
Hummel W, Schütte H, Kula M-R (1988) d-(−)-Mandelic acid dehydrogenase from Lactobacillus curvatus. Appl Microbiol Biotechnol 28: 433–439
Jallageas JC, Arnaud A, Galzy P (1980) Bioconversions of nitriles and their applications. Adv Biochem Eng 14: 1–32
Kakeya H, Sakai N, Sugai T, Ohta H (1991) Microbial hydrolysis as a potent method for the preparation of optically active nitriles, amides and carboxylic acids. Tetrahedron Lett 32: 1343–1346
Klepacka M, Rutkowski A (1982) The presence of nitriles in rapesead meal. Acta Aliment Pol 8: 3–10
Kobayashi M, Nagasawa T, Yamada H (1989) Nitrilase of Rhodococcus rhodochrous J1. Eur J Biochem 182: 349–356
La-Mer VK, Greenspan J (1934) Kinetics of the saponification of acetylated hydroxy acids. J Am Chem Soc 56: 1492–1499
Layh N, Stolz A, Knackmuss H-J, Effenberger F, Förster S (1991) Stereoselective hydrolysis of O-acetylmandelonitrile by bacterial nitrilases. VAAM-Tagung 1991, Freiburg/Br., p 137. In: BIO-forum 1/2. GIT Verlag, Darmstadt, p 60
Lockwood GB, Afsharypuor S (1986) Comparative study of the volatile aglucones of glucosinolates from in vivo and in vitro grown Descurainia sophia and Alyssum minimum using gas chromatography-mass spectrometry. J Chrom 356: 438–440
Maestracci M, Thiery A, Bui K, Galzy P (1984) Activity and regulation of an amidase (acylamide amidohydrolase, EC 3.5.1.4) with a wide substrate spectrum from a Brevibacterium sp. Arch Microbiol 138: 315–320
Mauger J, Nagasawa T, Yamada H (1990) Occurrence of a novel nitrilase, arylacetonitrilase, in Alcaligenes faecalis JM3. Arch Microbiol 155: 1–6
Mayaux JF, Cerbelaud E, Soubrier F, Faucher D, Petre D (1990) Purification, cloning, and primary structure of an enantiomer-selective amidase from Brevibacterium sp. strain R312: structural evidence for genetic coupling with nitrile hydratase. J Bacteriol 172: 6764–6773
Nagasawa T, Nanba H, Ryuno K, Talkeuchi K (1987) Nitrile hydratase of Ps. chlororaphis B23. Eur J Biochem 162: 691–698
Nagasawa T, Ryuno K, Yamada H (1988a) Occurrence of a cobalt-induced and cobalt-containing nitrile hydratase in Rhodococcus rhodochrous J1. Biochem Biophys Res Commun 155: 1008–1016
Nagasawa T, Kobayashi M, Yamada H (1988b) Optimum culture conditions for the production of benzonitrilase by Rhodococcus rhodochrous J1. Arch Microbiol 150: 89–94
Nagasawa T, Mauger J, Yamada H (1990) A novel nitrilase, arylacetonitrilase, of Alcaligenes faecalis JM3. Eur J Biochem 194: 765–772
Pfennig M, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55: 245–256
Robinson WG, Hook RH (1964) Ricine nitrilase. I. Reaction, product and substrate specificity. J Biol Chem 239: 4257–4262
Scherrer R (1984) Gram's staining reaction, Gram types and cell walls of bacteria. TIBS 9: 242–245
Tani Y, Kurihara M, Nishise H, Yamamoto K (1989) Biotransformation of dinitrile to mononitrile, a tranexamic acid intermediate, by Corynebacterium sp. Agric Biol Chem 53: 3143–3149
Utimoto K, Wakabayashi Y, Shisbiyama Y, Inoue M, Nozaki H (1981) 2-Alkoxy and 2,2-dialkoxy nitriles from acetals and orthoesters. Exchange of alkoxy into cyano group by means of cyanotrimethylsilane. Tetrahedron Lett 22: 4279–4280
Vaughan PA, Cheetham PSJ, Knowles CJ (1988) The utilization of pyridine carbonitriles and carboxamides by Nocardia rhodochrous LL100–21. J Gen Microbiol 134: 1099–1107
Watanabe I, Satoh Y, Enomoto K (1987) Sreening, isolation and taxonomical properties of microorganisms having acrylonitrile-hydrating activity. Agric Biol Chem 51: 3193–3199
Yamamoto K, Ueno Y, Otsubo K, Kawakami K, Komatsu KI (1990) Production of S(+)-Ibuprofen from a nitrile compound by Acinetobacter sp. strain AK226. Appl Environ Microbiol 56: 3125–3129
Yamamoto K, Oishi K, Fujimatsu I, Komatsu KI (1991) Production of R(−)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol 57: 3028–3032
Yamazaki Y, Maeda H (1986a) Enzymatic synthesis of optically pure (R)-(−)-mandelic acid and other 2-hydroxycarboxylic acids: screening for the enzyme, and its purification, characterization and use. Agric Biol Chem 50: 2621–2631
Yamazaki Y, Maeda H (1986b) Continuous production of (R)-(−)-mandelic acid in a bioreactor using the ultrafiltration method. Agric Biol Chem 50: 3213–3214
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Layh, N., Stolz, A., Förster, S. et al. Enantioselective hydrolysis of O-acetylmandelonitrile to O-acetylmandelic acid by bacterial nitrilases. Arch. Microbiol. 158, 405–411 (1992). https://doi.org/10.1007/BF00276300
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276300