Abstract
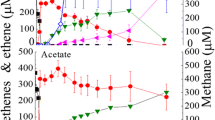
An anaerobic enrichment culture degraded 1 mol of acetone to 2 mol of methane and 1 mol of carbon dioxide. Two microorganisms were involved in this process, a filament-forming rod similar to Methanotrix sp. and an unknown rod with round to slightly pointed ends. Both organisms formed aggregates up to 300 μm in diameter. No fluorescing bacteria were observed indicating that hydrogen or formate-utilizing methanogens are not involved in this process. Acetate was utilized in this culture by the Methanothrix sp. Inhibition of methanogenesis by bromoethanesulfonic acid or acetylene decreased the acetone degradation rate drastically and led to the formation of 2 mol acetate per mol of acetone. Streptomycin completely inhibited acetone degradation, and neither acetate nor methane was formed. 14CO2 was incorporated exclusively into the C-1 atom of acetate indicating that acetone is degraded via carboxylation to an acetoacetate residue. It is concluded that acetone is degraded by a coculture of an eubacterium and an acetate-utilizing methanogen and that acetate is the only intermediate transferred between both. The energetical problems of the eubacterium converting acetone to acetate are discussed.
Similar content being viewed by others
References
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiontic association of two species of bacteria. Arch Mikrobiol 59:20–31
Davies R, Stephenson M (1941) Studies on the acetone-butyl-alcohol fermentation. I. Nutritional and other factors involved in the preparation of active suspensions of Clostridium acetobutylicum (Weizmann). Biochem J 35:1320–1331
Dimroth P (1982) Decarboxylation and transport. Bioscience Reports 2:849–860
Dolfing J, Mulder JW (1985) Comparison of methane production rate and coenzyme F420 content of methanogenic consortia in anaerobic granular sludge. Appl Environ Microbiol 49:1142–1145
Dolfing J, Griffioen A, Neerven ARW van, Zevenhuizen LPTM (1985) Chemical and bacteriological composition of granular methanogenic sludge. Can J Microbiol 31:744–750
Fathepure BZ (1983) Isolation and characterization of an acetoclastic methanogen from a biogas digester. FEMS Microbiol Lett 19:151–156
Flett RJ, Hamilton RD, Campbell NER (1976) Aquatic acetylene reduction techniques: solution to several problems. Can J Microbiol 22:43–51
Fuchs G, Stupperich E, Eden G (1980) Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch Microbiol 128:64–71
Gunsalus RP, Romesser JA, Wolfe RS (1978) Preparation of coenzyme M analogues and their activity in the methylcoenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochem 17:2374–2377
Hilpert W, Schink B, Dimroth P (1984) Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J 3:1665–1670
Landau BR, Brunengraber H (1987) The role of acetone in the conversion of carbohydrates. Trends Biochem Sci 12:113–114
Lukins HB, Foster JW (1963) Methylketone metabolism in hydrocarbon utilizing mycobacteria. J Bacteriol 85:1074–1087
Mazé P (1915) Ferment forménique. Fermentation forménique de l'aceton. Procédé du culture simple du ferment forménique. Compt Rendue Soc Biol 78:395–405
Northrop JH, Ashe LH, Senior JK (1919) Biochemistry of Bacillus acetoethylicum with reference to the formation of acetone. J Biol Chem 39:1–21
Patel GB (1984) Characterization and nutritional properties of Methanothrix concilii sp. nov., a mesophilic acetoclastic methanogen. Can J Microbiol 30:1383–1396
Pecher T, Böck A (1981) In vivo susceptibility of halophilic and methanogenic organisms to protein synthesis inhibitors. FEMS Microbiol Lett 10:295–297
Pfennig N (1978) Rhodocyclus purpureus gen. nov. and sp. nov., a ring shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Schardinger F (1905) Bacillus macerans, ein Aceton-bildender Rottebacillus. Zbl Bakteriol Parasitenkd Infektionskr Hyg Abt II 14:772–781
Schink B, Pfennig N (1982) Fermentation of trihydroxybenzenes by Pelobacter acidigallicic gen. nov. sp. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch Microbiol 133:195–201
Simon H, Floss H (1967) Anwendung von Isotopen in der organischen Chemie and Biochemie, vol 1. Springer, Berlin Heidelberg New York, pp 23–27
Sprott GD, Jarell KF, Shaw KM, Knowles R (1982) Acetylene as an inhibitor of methanogenesis. J Gen Microbiol 128:2453–2462
Stieb M, Schink B (1986) Anaerobic degradation of isovalerate by a defined methanogenic coculture. Arch Microbiol 144:291–295
Stryer L (1981) Biochemistry, 2nd edn. WH Freeman and Company, San Francisco, USA
Symons GE, Buswell AM (1933) The methane fermentation of carbohydrates. J AM Chem Soc 55:2028–2036
Taylor DG, Trudgill PW, Gripps RE, Harris PR (1980) The microbial metabolism of acetone. J Gen Microbiol 118:159–170
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Westheimer FH (1963) The mechanism of the enzymic decarboxylation of acetoacetic acid. Proc Chem Soc USA 253–261
Widdel F (1986) Growth of methanic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol 51:1056–1062
Widdel F (1987) Microbiology and ecology of sulfate- and sulfurreducing bacteria. In: Environmental biology of anaerobes, Zehnder AJB (ed) (in press)
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of a new sulfate-reducer enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol 129:395–400
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Wikén T (1940) Untersuchungen über Methangärung und die dabei wirksamen Bakterien. Arch Mikrobiol 11:312–317
Zehnder AJB, Huser BA, Brock TD, Wuhrmann K (1980) Characterization of an acetate-decarboxylating, nonhydrogen-oxidizing methane bacterium. Arch Microbiol 124:1–11
Zinder SH, Anguish T, Lobo AL (1987) Isolation and characterization of a thermophilic strain of Methanothrix. Arch Microbiol 146:315–322
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Platen, H., Schink, B. Methanogenic degradation of acetone by an enrichment culture. Arch. Microbiol. 149, 136–141 (1987). https://doi.org/10.1007/BF00425079
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425079