Abstract
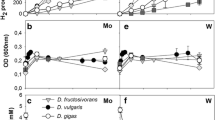
During growth on glycerol two marine Desulfovibrio strains that can grow on an unusually broad range of substrates contained high activities of glycerol kinase, NAD(P)-independent glycerol 3-phosphate dehydrogenase and the other enzymes necessary for the conversion of dihydroxyacetone phosphate to pyruvate. Glycerol dehydrogenase and a specific dihydroxyacetone kinase were absent. During growth on dihydroxyacetone, glycerol kinase is involved in the initial conversion of this compound to dihydroxyacetone phosphate which is then further metabolized. Some kinetic properties of the partially purified glycerol kinase were determined. The role of NAD as electron carrier in the energy metabolism during growth of these strains on glycerol and dihydroxyacetone is discussed.
Glycerol also supported growth of three out of four ‘classical’ Desulfovibrio strains tested. D. vulgaris strain Hildenborough grew slowly on glycerol and contained glycerol kinase, glycerol 3-phosphate dehydrogenase and enzymes for the dissimilation of dihydroxyacetone phosphate. In D. gigas which did not grow on glycerol the enzymes glycerol kinase and glycerol 3-phosphate dehydrogenase were absent in lactate-grown cells.
Similar content being viewed by others
Abbreviations
- DHA:
-
dihydroxyacetone
- DHAP:
-
dihydroxyacetone phosphate
- G3P:
-
glycerol 3-phosphate
- GAP:
-
glyceraldehyde 3-phosphate
- 3-PGA:
-
3-phosphoglycerate
- 2-PGA:
-
2-phosphoglycerate
- 2,3-DPGA:
-
2,3-diphosphoglycerate
- PEP:
-
phosphoenolpyruvate
- DH:
-
dehydrogenase
- GK:
-
glycerol kinase
- DHAK:
-
dihydroxyacetone kinase
- TIM:
-
triosephosphate isomerase
- PGK:
-
3-phosphoglycerate kinase
- PK:
-
pyruvate kinase
- LDH:
-
lactate dehydrogenase
- DTT:
-
dithiotreitol
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid
- PIPES:
-
piperazine-1,1′-bis(2-ethane sulfonic acid)
- BV2+/BV+ :
-
oxidized/reduced benzylviologen
- PMS:
-
phenazine methosulfate
- DCPIP:
-
2,6-dichlorophenolindophenol
- MTT:
-
3-(4′,5′-dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide
References
Abeles RH, Brownstein AM, Randles CH (1960) β-Hydroxypropionaldehyde, an intermediate in the formation of 1,3-propane-diol by Enterobacter aerogenes. Biochim Biophys Acta 41:530–531
Ameyama M, Shinagawa E, Matsushita K, Adachi O (1985) Solubilization, purification and properties of membrane-bound glycerol dehydrogenase from Gluconobacter industrius. Agric Biol Chem 49:1001–1010
Baker FD, Papiska HR, Campbell LL (1962) Choline fermentation by Desulfovibrio desulfuricans. J Bacteriol 84:973–978
Bergmeyer HU, Gawehn K, Grassl M (1974) Enzymes as biological reagents. In: Bergmeyer (ed) Methods of enzymatic analysis, vol 1. Academic Press, Inc. New York, pp 425–522
Bradford MM (1976) A rapid sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brehe JE, Burch HB (1976) Enzymatic assay for glutathione. Anal Biochem 74:189–197
Cooper RA (1984) Metabolism of methylglyoxal in microorganisms. Ann Rev Microbiol 38:49–68
Cooper RA, Anderson A (1970) The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett 11:273–276
Forage RG, Foster MA (1982) Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12 dependent glycerol and diol dehydratases. J Bacteriol 149:413–419
Goa J (1953) A microbiuret method for protein determination: determination of total protein in the cerebrospinal fluid. Scand J Clin Lab Invest 5:218–222
Hardy JA (1981) The enumeration, isolation and characterization of sulphate-reducing bacteria from North Sea waters. J Appl Bacteriol 51:505–516
Higgins IJ, Turner JM (1969) Enzymes of methylglyoxal metabolism in a Pseudomonad which rapidly metabolizes aminoacetone. Biochim Biophys Acta 184:464–467
Hopper DJ, Cooper RA (1971) The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett 13:213–216
Howard BH, Hungate RE (1976) Desulfovibrio of the sheep rumen. Appl Environ Microbiol 32:598–602
Jin RZ, Forage RG, Lin ECC (1982) Glycerol kinase as a substitute for dihydroxyacetone kinase in a mutant of Klebsiella pneumoniae. J Bacteriol 152:1303–1307
Johnson BC, Stroinski A, Schneider Z (1975) Glycerol dehydratase from Aerobacter aerogenes. In Wood ed Methods in enzymology, vol 42C. Academic Press, New York London, pp 315–323
Laanbrock HJ, Abee T, Voogd IL (1982) Alcohol conversions by Desulfobulbus propionicus Lindhorst in the presence and absence of sulfate and hydrogen. Arch Microbiol 133:178–184
Lang E, Lang H (1972) Spezifische Farbreaktion zum direkten Nachweis der Ameisensäure. Fresenius Z Anal Chem 260:8–10
Lin ECC (1976) Glycerol dissimilation and its regulation in bacteria. Ann Rev Microbiol 30:535–578
Murata K, Fukuda Y, Simosaka M, Watanabe K, Saikusa T, Kimura A (1985) Purification and characterization of NADPH-dependent methylglyoxal-reducing enzyme from Saccharomyces cerevisiae. Eur J Biochem 151:631–636
Nanninga HJ, Gottschal JG (1986) Isolation of a sulfate-reducing bacterium growing with methanol. FEMS Microbiol Ecol 38:125–130
Nishise H, Tani Y, Yamada H (1985) Alternation of the dissimilation pathway for glycerol and amplification of glycerol dehydrogenase in mutant strains of Cellulomonas sp. NT3060. Agric Biol Chem 49:3115–3122
Odom JM, Peck HD (1981) Localization of dehydrogenases, reductases and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 147:161–169
Peck HD, LeGall J (1982) Biochemistry of dissimilatory sulphate reduction. Phil Trans R Soc Lond B 298:443–466
Pfennig N, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55:245–256
Postgate JR (1984) The sulphate-reducing bacteria. Cambridge University Press, Cambridge
Riddle V, Lorenz FW (1968) Nonenzymic, polyvalent anioncatalyzed formation of methylglyoxal as an explanation of its presence in physiological systems. J Biol Chem 243:2718–2724
Skyring GW, Jones HE, Goodchild D (1977) The taxonomy of some new isolates of dissimilatory sulfate-reducing bacteria. Can J Microbiol 23:1415–1425
Spector DL, Pizer LI (1975) l-Glycerol-3-phosphate dehydrogenase from Escherichia coli. In: Wood WA (Ed) Methods in enzymology, vol 41 B. Academic Press, New York London, pp 249–254
Stams AJM, Hansen TA, Skyring GW (1985) Utilization of amino acids as energy substrates by two marine Desulfovibrio strains. FEMS Microbiol Ecol 31:11–15
Stams AJM, Hansen TA (1986) Metabolism of l-alanine in Desulfotomaculum ruminis and two marine Desulfovibrio strains. Arch Microbiol 145:277–279
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Stams AJM, Veenhuis M, Weenk GH, Hansen TA (1983) Occurrence of polyglucose as a storage polymer in Desulfovibrio species and Desulfobulbus propionicus. Arch Microbiol 136: 54–59
Taylor DG, Trudgill PW, Cripps RE, Harris PR (1980) The microbial metabolism of acetone. J Gen Microbiol 118:159–170
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Thorner JW (1975) Glycerol kinase. In: Wood WA (ed) Methods in enzymology, vol 42C. Academic Press, New York London, pp 148–156
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. I. Quantitative measurement of growing cells of Chromatium okenii. Antonie van Leeuwenhoek J Microbiol Serol 30:225–238
Widdel F (1980) Anaerober Abbau von Fettsäuren und Benzoesäure durch neu isolierte Arten sulfatreduzierender Bakterien. Ph D Thesis, University of Göttingen
Wieland O (1974) Glycerol UV method. In: Bergmeyer HU (ed) Methods in enzymatic analysis. Academic Press, New York, pp 1404–1409
Yamada H, Nagao A, Nishihe H, Tani Y (1982) Glycerol dehydrogenase from Cellulomonas sp. NT3060: Purification and characterization. Agric Biol Chem 46:2333–2339
Zee R van der, Welling GW (1984) A personal-computer based gradient system for high-performance liquid chromatography with low-pressure mixing. J Chromatography 292:412–417
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kremer, D.R., Hansen, T.A. Glycerol and dihydroxyacetone dissimilation in Desulfovibrio strains. Arch. Microbiol. 147, 249–256 (1987). https://doi.org/10.1007/BF00463484
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00463484