Summary
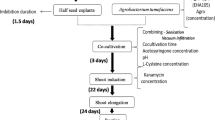
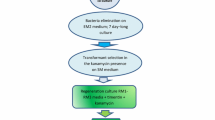
A simplified protoplast regeneration system for Vigna aconitifolia was developed. A plating efficiency of 60% was obtained using mesophyll protoplasts from 10-day-old seedlings. By co-cultivation of protoplasts with Agrobacterium tumefaciens containing the Ti plasmid derivative pGV 3850∶1103 neo kanamycin-resistant colonies were obtained; 23% of the transformed lines showed expression of the nonselected co-transferred nopaline synthase gene. Transformation was confirmed by Southern blot analysis using a nonradioactive detection system. The plant cultivar used was an important factor in determining transformation frequencies since one of the cultivars had an 85 fold higher transformation rate than the other.
Similar content being viewed by others
References
Binding H (1974) Regeneration von haploiden und diploiden Pflanzen aus Protoplasten von Petunia hybrida. Z Pflanzenphysiol 74:327–356
Czernilofsky AP, Hain R, Herrera-Estrella L, Lörz H, Goyvaerts E, Baker B, Schell J (1986) Fate of selectable marker DNA integrated into the genome of Nicotiana tabacum. DNA 5:101–113
Garcia JA, Hille J, Goldbach R (1986) Transformation of cowpea Vigna unguiculata cells with an antibiotic resistance gene using a Ti plasmid derived vector. Plant Sci 44:37–46
Hain R, Stabel P, Czernilofsky AP, Steinbiss HH, Herrera-Estrella L, Schell J (1985) Uptake, integration, expression and genetic transmission of a selectable chimaeric gene by plant protoplasts. Mol Gen Genet 199:161–168
Halperin W (1986) Attainment and retention of morphogenetic capacity in vitro. In: Vasil I (ed) Cell culture and somatic cell genetics of plants, vol 3. Academic Press, Orlando, pp3–47
Herrera-Estrella L, Depicker A, Van Montagu M, Schell J (1983) Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303:209–213
Köhler F, Golz C, Eapen S, Kohn H, Schieder O (1987a) Stable transformation of moth bean Vigna aconitifolia via direct gene transfer. Plant Cell Rep 6:313–317
Köhler F, Golz C, Eapen S, Schieder O (1987 b) Influence of plant cultivar and plasmid DNA on transformation rates in tobacco and moth bean. Plant Sci (in press)
Lörz H, Baker B, Schell J (1985) Gene transfer to cereal cells mediated by protoplast transformation. Mol Gen Genet 199:178–182
Marton L, Wullems GJ, Molendijk L, Schilperoort RA (1979) In vitro transformation of cultured cells from Nicotiana tabacum by Agrobacterium tumefaciens. Nature 277:129–131
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–496
Otten L, Schilperoort RA (1978) A rapid microscale method for the detection of lysopine and nopaline dehydrogenase activities. Biochem Biophys Acta 527:497–500
Paszkowski J, Pisan B, Shillito RD, Hohn T, Hohn B, Potrykus I (1984) Direct gene transfer to plants. EMBO J 3:2717–2722
Potrykus I, Saul MW, Petruska J, Paszkowski J, Shillito RD (1985) Direct gene transfer to cells of a graminaceous monocot. Mol Gen Genet 199:183–188
Schieder O (1984) Isolation and culture of protoplasts: Datura. In: Vasil I (ed) Cell culture and somatic cell genetics of plants, vol I. Academic Press, Orlando, pp 350–355
Shahin EA, Spielmann A, Sukhapinda K, Simpson RB, Yashar M (1986) Transformation of cultivated alfalfa using disarmed Agrobacterium tumefaciens. Crop Sci 26:1235–1238
Shekhawat NS, Galston AW (1983) Isolation, culture, and regeneration of moth bean Vigna aconitifolia leaf protoplasts. Plant Sci Lett 32:43–51
Shillito RD, Paszkowski J, Potrykus I (1983) Agarose plating and bead type culture technique enable and stimulate development of protoplast-derived colonies in a number of plant species. Plant Cell Rep 2:244–247
Shillito RD, Saul MW, Paszkowski J, Müller M, Potrykus I (1985) High efficiency direct gene transfer to plants. Biotechnol 3:1099–1103
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Wullems GJ, Molendijk L, Ooms G, Schilperoort RA (1981) Differential expression of crown gall tumor markers in transformants obtained after in vitro Agrobacterium tumefaciens induced transformation of cell wall regenerating protoplasts derived from Nicotiana tabacum. Proc Natl Acad Sci USA 78:4344–4348
Author information
Authors and Affiliations
Additional information
Communicated by G. Wenzel
On deputation from: Bhabha Atomic Research Centre, Bombay, India, under the Indo-FRG Bilateral Programme
Rights and permissions
About this article
Cite this article
Eapen, S., Köhler, F., Gerdemann, M. et al. Cultivar dependence of transformation rates in moth bean after co-cultivation of protoplasts with Agrobacterium tumefaciens . Theoret. Appl. Genetics 75, 207–210 (1987). https://doi.org/10.1007/BF00249165
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00249165