Abstract
Purpose. To develop a pharmacokinetic model for tenidap and to identify important relationships between the pharmacokinetic parameters and available covariates.
Methods. Plasma concentration data from several phase I and phase II studies were used to develop a pharmacokinetic model for tenidap, a novel anti-rheumatic drug. An appropriate pharmacokinetic model was selected on the basis of individual nonlinear regression analyses and an EM algorithm was used to perform a nonlinear mixed-effects analysis. Scatter plots of posterior individual pharmacokinetic parameters were used to identify possible covariate effects.
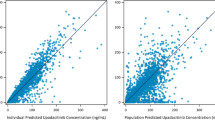
Results. Predicted responses were in good agreement with the observed data. A bi-exponential model with zero order absorption was subsequently used to develop the mixed-effects model. Covariate relationships selected on the basis of differences in the objective function, although statistically significant, were not particularly strong.
Conclusions. The pharmacokinetics of tenidap can be described by a bi-exponential model with zero order absorption. Based on differences in the log-likelihood, significant covariate-parameter relationships were identified between smoking and CL, and between gender and Vss and CLd. Simulated sparse data analyses indicated that the model would be robust for the analysis of sparse data generated in observational studies.
Similar content being viewed by others
REFERENCES
T. Pullar. The pharmacokinetics of tenidap sodium: introduction. Br. J. Clin. Pharmac. 39:1S–2S (1995).
W. D. Blackburn, H. M. Prupas, J. C. Silverfield, J. E. Poiley, J. R. Caldwell, R. L. Collins, M. J. Miller, D. H. Sikes, H. Kaplan, R. Fleischmann, C. D. Scoville, J. E. Rutstein, E. R. Hurd, J. S. Louie, A. D. Bankhurst, A. L. Weaver, A. I. Sebba, D. J. Appelrouth, N. P. Hudson, G. V. Gordon, R. D. Gordon, C. L. Ludivico, M. C. Austin, K. M. Sanders, P. T. Schuette, R. A. Moidel, A. R. Kraska, N. Ting, W. R. Shanahan, and L. D. Loose. Tenidap in rheumatoid arthritis. A 24-week double-blind comparison with hydroxychloroquine-plus-piroxicam, and piroxicam alone. Arthritis Rheum. 38:1447–1456 (1995).
R. Luqmani, C. Gordon, and P. Bacon. Clinical pharmacology and modification of autoimmunity and inflammation in rheumatoid disease. Drugs 47:259–285 (1994).
M. J. Gardner, K. D. Wilner, R. A. Hansen, H. G. Fouda, and G. F. McMahon. Single and multiple dose pharmacokinetics of tenidap sodium in healthy subjects. Br. J. Clin. Pharmac 39:11S–15S (1995).
J. R. Caldwell, D. S. Kirby, M. J. Gardner, and R. A. Hansen. The effects of age and gender on the pharmacokinetics of tenidap sodium in patients with rheumatoid arthritis and osteoarthritis. Br. J. Clin. Pharmac. 39:3S–9S (1995).
M. J. Avery, D. Y. Mitchell, F. C. Falkner, and H. G. Fouda. Simultaneous determination of tenidap and its stable isotope analog in serum by high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Biol. Mass Spectrometry 21:353–357 (1992).
SIPHAR Users Manual. SIMED—Centres D'Etudes et de Recherches en Statistiques et Informatique Medicales, 1988.
L. Aarons. The estimation of population pharmacokinetic parameters using an EM algorithm. Comp. Meth. Prog. Biomed. 41:9–16 (1993).
J. G. Wagner. Fundamentals of clinical pharmacokinetics. Drug Intelligence Publications Inc., 1979.
V. K. Piotrovskii. The use of Weibull distribution to describe the in vivo absorption kinetics. J. Pharmacokin. Biopharm. 15:681–686 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, L., Aarons, L. & Coates, P. A Pharmacokinetic Model for Tenidap in Normal Volunteers and Rheumatoid Arthritis Patients. Pharm Res 16, 1608–1615 (1999). https://doi.org/10.1023/A:1018969024101
Issue Date:
DOI: https://doi.org/10.1023/A:1018969024101