Abstract
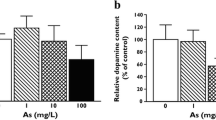
Our previous studies have shown that exposure to low levels of Pb results in significant reductions in dopamine (DA) and its metabolites (3,4-dihyroxyphenylacetic acid, DOPAC and homovanillic acid, HVA) in nucleus acumbens (NA). This area of brain receives dopaminergic projections from the ventral tegmentum and is considered vital in manifestation of many behavioral responses. Similarly, basal and K+-induced release of DA was found significantly reduced in the Pb-exposed rats as compared to the controls in this brain region. Additional studies indicated that acute infusion of Pb in nucleus acumbens caused significant release of DA. Based on these observations it was postulated that the reductions in DA contents and in the basal and stimulus-induced release of DA in NA were manifestations of attenuated dopaminergic activity in this brain region. However, the mechanism of this attenuation is not yet clear. Studies reported here were designed to evaluate the role of a key regulatory enzy me in biosynthesis of DA, i.e. tyrosine hydroxylase (TH) in Pb-induced reductions in dopaminergic activity. The results of these studies indicated that 50 and 500 ppm Pb produced 22.8 and 56% inhibition of TH activity in vitro respectively, and that the enzyme activity was reduced to 43% in rats exposed to 50 ppm lead for 30 days as compared to the controls. The alterations in TH activity in Pb-exposed animals were further confirmed by Western blot analysis. Collectively, these results suggest that Pb-induced inhibition of TH activity in rat brain may contribute to the reductions in dopaminergic activity observed in Pb-exposed animals. (Mol Cell Biochem 175: 137–141, 1997)
Similar content being viewed by others
References
Kala SV, Jadhav AL: Region-specific alterations in dopamine and serotonin metabolism in brains of rats exposed to low levels of Lead. Neuro Toxicol 16(2): 297–308, 1995
Kala SV, Jadhav AL: Low level Lead exposure decreases in vivo release of dopamine in the rat nucleus accumbens: a microdialysis study. J Neurochem 65: 1631–1635, 1995
Jadhav AL, Kala SV: Mechanism of attenuation of dopaminergic activity in nucleus accumbens of rats exposed to low levels of lead. In: P Callery, J Corbella, JL Damingo, JC Etienne, JM Llobet (eds). Metal Ions in Biology and Medicine. John Libbey Eurotext, Paris, 1995, pp 320–322
Meredith PA, McIntosh MJ, Petty MA, Reid JL: Effects of lead exposure on rat brain catecholaminergic neurochemistry. Comp Biochem Physiol 89C(2): 215–219, 1988
McIntosh MJ, Meredith PA, Petty MA, Reid JL: Influence of lead exposure on catecholamine metabolism in discrete rat brain nuclei. Comp Biochem Physiol 89C(2): 211–213, 1988
Cory-Slechta DA, Widzowski DV, Pokora MJ: Functional alterations in dopamine systems assessed using drug discrimination procedures. Neuro Toxicol 14: 105–114, 1993
Lasley SM, Greenland RD, Minnema DJ, Michaelson IA: Influence of chronic inorganic lead exposure on regional dopamine and 5-hydroxytryptamine turnover in rat brain. Neurochem Res. 9: 1675–1688, 1984
Lasley SM: Regulation of dopaminergic activity, but not tyrosine hydroxylase, is diminished after chronic inorganic lead exposure. Neuro Toxicol 13: 625–636, 1992
Lasley SM, Lane JD: Diminished regulation of mesolimbic dopaminergic activity in rat after chronic inorganic lead exposure. Toxicol App Pharmacol 95: 474–483, 1988
Cory-Slechta DA, Weiss B, Cox C: Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol App Pharmacol 78: 291–299, 1985
Knowles M: The determination of lead and cadmium in blood and manganese and aluminum in serum. Varian Instrs At Work, 1987, p AA-76
Waymire JC, Bjur R, Weiner N: Assay of tyrosine hydroxylase by coupled decarboxylation of dope formed from 14C-L-tyrosine. Anal Biochem 43: 588–600, 1971
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Pro Natl Acad Sci USA 76: 4350–4354, 1979
Markey KA, Kondo S, Shenkrnan L, Goldstein M: Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17: 79–85, 1980
Thibault J, Vidal D, Gros F: In vitro translation of mRNA from rat pheochromocytoma tumors, characterization of tyrosine hydroxylase. Biochem Biophys Res Commun 99: 960–968, 1981
Bellinger D, Leviton A, Waternaux C, Needlman HL, Rabinowitz M: Low level Lead exposure, social class and infant development. Neuro Toxicol Teratol 10: 497–503, 1989
Needleman HL: The current status of childhood low level lead toxic ity. Neuro Toxicol 14: 161–166, 1993
Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB: Bone lead and delinquent behavior. JAMA 275(5): 363–369, 1996
Michel PP, Vyas S, Anglade P, Agid Y: Morphological and molecular characterization of the response of differentiated PC12 cells to calcium stress. Eur J Neuroscience 6(4): 577–586, 1994
Simons TJ: Lead calcium interactions in cellular lead toxicity. Neuro Toxicol 14(2–3): 77–86, 1993
Goldstein GW: Evidence that lead acts as a calcium substitute in second messenger metabolism. Neuro Toxicol 14(2–3): 77–86, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jadhav, A.L., Ramesh, G.T. Pb-induced alterations in tyrosine hydroxylase activity in rat brain. Mol Cell Biochem 175, 137–141 (1997). https://doi.org/10.1023/A:1006891830182
Issue Date:
DOI: https://doi.org/10.1023/A:1006891830182