Abstract
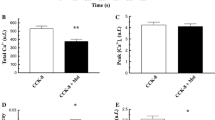
This study investigates the effect of magnesium (Mg2+) on the secretory responses and the mobilization of calcium (Ca2+) and Mg2+ evoked by cholecystokinin-octapeptide (CCK-8) in the exocrine rat pancreas. In the isolated intact perfused pancreas CCK-8 (10−10 M) produced marked increases in juice flow and total protein output in zero and normal (1.1 mM) extracellular Mg2+ [Mg2+]o compared to a much reduced secretory response in elevated (5 mM and 10 mM) [Mg2+]o Similar effects of perturbation of [Mg2+]o on amylase secretion and 45Ca2+ uptake (influx) were obtained in isolated pancreatic segments. In pancreatic acinar cells loaded with the fluorescent bioprobe fura-2 acetomethylester (AM), CCK-8 evoked marked increases in cytosolic free Ca2+ concentration [Ca2+]i in zero and normal [Mg2+]o compared to a much reduced response in elevated [Mg2+]o Pretreatment of acinar cells with either dibutyryl cyclic AMP (DB2 cAMP) or forskolin had no effect on the CCK-8 induced changes in [Ca2+]i. In magfura-2-loaded acinar cells CCK-8 (10−8 M) stimulated an initial transient rise in intracellular free Mg2+ concentration [Mg2+]i followed by a more prolonged and sustained decrease. This response was abolished when sodium Na+ was replaced with N-methyl-D-glucamine (NMDG). Incubation of acinar cells with 10 mM Mg2+ resulted in an elevation in [Mg2+]i. Upon stimulation with CCK-8, [Mg2+]i. decreased only slightly compared with the response obtained in normal [Mg2+]o. CCK-8 caused a net efflux of Mg2+ in pancreatic segments; this effect was abolished when extracellular sodium [Na+]o was replaced with either NMDG or choline. The results indicate that Mg2+ can regulate CCK-8-evoked secretory responses in the exocrine pancreas possibly via Ca2+ mobilization. Moreover, the movement of Mg2+ in pancreatic acinar cells is dependent upon extracellular Na+.
Similar content being viewed by others
References
Naminga LB: Calculation of free magnesium, calcium and potassium in muscle. Biochim Biophysic Acta 54: 338–344, 1961
Alvares C Jefmans FJ, Gamino SM, Giraldez F, Gonzalez-Serratos H: Intracellular free magnesium in frog muscle fibres measured with ionselective micro-electrodes. J Physiol (Lond), 378: 461–483, 1986
Wacker WEC: The biochemistry and physiology of magnesium. Ann NY Acad Sci 161: 717–726, 1968
Flatman PW: Magnesium transport across cell membranes. J. Membrane Biol 80: 1–14, 1984
Squire LV, Petersen OH: Modulation of Ca2+ and voltage-activated K+ channels by internal Mg2+ in salivary acinar cells. Biochim Biophysica Acta 899: 171–175, 1987
Agus ZS, Kelepouris E, Dukes I, Morad M: Cytosolic magnesium modulates calcium channel activity in mammalian ventricular cells. Am J Physiol 256: C452-C455, 1989
Flatman PW, Smith LM: Magnesium transport in ferret red cells. J Physiol (Lond) 431: 11–25, 1990
Francis LP, Lennard R, Singh J: Mechanism of action of magnesium on acetylcholine-evoked secretory responses in isolated rat pancreas. Exp Physiol 75: 669–680, 1990
Flatman PW: The role of magnesium in regulating ion transport. In: N.J. Birch (ed.). Magnesium and Cell. Academic Press, London, 1993, pp 197–216
Neilsen SP, Petersen OH: Transport of calcium in the perfused submandibular gland of the cat. J Physiol (Lond) 223: 685–697, 1972
Sullivan JF, Burch RE, Magee DF: Enzyme activity and divalent cation content of pancreatic juice. Am J Physiol 226: 1420–1423, 1974
Baker PF, Knight DE: Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature 276: 620–622, 1978
Lennard R, Singh J: Secretagogue-evoked changes in intracellular free magnesium concentrations in rat pancreatic acinar cells. J. Phvsiol (Lond) 435: 483–492, 1991
Lennard R, Singh J: Effects of secretagogues on intracellular free calcium and magnesium concentrations in rat pancreatic acinar cells. General Pharmacol 23: 903–908, 1992
Streb H, Schulz I: Regulation of cytosolic free Ca2+ concentration in acinar cells of rat pancreas. Am J Physiol 245: G6347-G6357, 1983
Shears SB: Metabolism of the inositol phosphates produced upon receptor activation. Biochem J 260: 313–324, 1989
Brown GR, Richardson AE, Dormer RL: The role of a (Ca2+ + Mg2+). ATPase of the rough endoplasmic reticulum in regulating intracellular Ca2+ during cholinergic stimulation of rat pancreatic acini. Biochim Biophysic Acta 902: 87–92, 1987
Galvan A, Lucas M: Ionic and substrate requirements of high affinity calcium-pumping ATPase in endoplasmic reticulum of pancreas. International J Biochem 19: 987–993, 1987
Osipchuk YV, Wakui M, Yule DI, Gallacher DV, Petersen OH: Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+ simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO 9: 697–704, 1990
Meissner G, Darling E, Eveleth J: Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+ and adenine nucleotides. Biochemistry 25: 236–244, 1986
Wisdom DM, Singh J, Salido GM, Camello PJ: Mechanism of action of magnesium on cholecystokinin-octapeptide-evoked secretory responses in the isolated exocrine rat pancreas. J. Physiol (Lond) 467: 105P, 1993
Kanno T: Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J. Physiol (Lond) 226: 353–371, 1972
Bradford MM: A rapid and sensitive method for quantification of nanogram quantities of protein utilising the principle of protein dye binding. Analytical Biochem 72: 248–254, 1976
Rindernecht H, Marbach EP: A new automated method for the determination of serum a-amylase. Clin Med Acta 29: 107–110, 1970
Grynkiewicz G, Poenie M, Tsien RY: A new generation of calcium indicators with greatly improved fluorecence properties. J Biol Chem 260: 3440–3450, 1985
Raju B, Murphy E, Levy LA, Hall RD, London RE: A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol 256, C540-C548, 1989
Case R M, Clausen T: The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol, (Lond) 235: 75–102, 1973
Lennard R, Francis L P, Singh J: Extracellular magnesium regulates acetylcholine-evoked amylase secretion and calcium mobilization in rat pancreatic acinar cells. Quart J Exp Physiol 74: 747–750, 1989
Berridge MJ: Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993
Wakui M, Potter VLB, Petersen OH: Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature 339: 317–320, 1989
Capurro DE, Dormer RL: Ca2+-induced Ca2+ release from endoplasmic reticular membranes of rat pancreas. Digestion 43: 37, 1989
Günther T, Vormann J: Magnesium efflux is accompanied by an amiloride sensitive Na+-Mg2+ anteport. Biochem Biophys Res Commun 130: 540–545, 1985
Fry CH: Measurement and control of intracellular magnesium ion concentration in guinea pig and ferret myocardium. Magnesium 5: 306–331, 1986
Buri A, Chen S, Fry CH, Illner H, Kickenweiz E, McGuigan JAS, Noble D, Powell T, Twist VW: The regulation of intracellular Mg2+ in guinea-pig heart studied with Mg2+-selective microelectrodes and fluorochromes. Physiol 78: 221–233, 1993
Gunther T, Vormann J, Höllriegl V: Characterization of Na+ dependent Mg2+ efflux from Mg2+-loaded rat erythrocytes. Biochim Biophysic acta 1023: 455–461, 1990
Gonzales A, Pariente JA, Salido GM, Singh J, Wisdom DM: Effects of different secretagogues on magnesium mobilisation in isolated rat pancreatic acinar cells. J Physiol (Lond) 482P: 33P, 1995
Wisdom DM, Singh J, Domschke W, Stoll R, Mooren F Ch: Regulation of cholecystokinin — octapeptide evoked magnesium transport in single mouse pancreatic acinar cells. J Physiol (Lond.) 485P: 36P, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wisdom, D.M., Salido, G.M., Baldwin, L.M. et al. The role of magnesium in regulating CCK-8-evoked secretory responses in the exocrine rat pancreas. Mol Cell Biochem 154, 123–132 (1996). https://doi.org/10.1007/BF00226780
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226780