Abstract
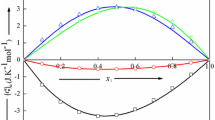
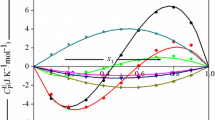
Excess molar heat capacities C E P at constant pressure and excess molar volumes VE have been determined, as a function of mole fraction x1 at 25°C and atmospheric pressure, for 10 binary liquid mixtures containing either trichloromethane (series I) with C6H5CH3, or C6H5Cl, or C5H5N, or CH3COCH3, or C6H5NO2; 1,4-dioxane (series II) with (C2H5)3N, or (CH3)2CHOCH(CH3)2, or (CH3 2SO); or diisopropyl ether (di-1-methylethyl ether) (series III) with (C2H5)3N, or CHCl3. The dipole momentsp (10−30C-m) of the substances range from nearly 0 to 14.1 for nitrobenzene. The C E P of series I and III are all positive, with C E P (x1=0.5) (J-K−1-mol−1) ranging from 1.04 for {x1CHCl3+x2C6H5Cl} to 16.66 for {x1(CH3)2CHOCH(CH3)2+x2CHCl3}. In series II, the C E P are positive and small for {x11,4-C4H8O2+x2(CH3)2CHOCH(CH3)2}, S-shaped and small for {x11,4-C4H8O2+x2(C2H5)3N}, and negative and small for {x11,4-C4H8O2+x2(CH3)2SO}. The excess volumes are small and positive for {x1CHCl3+x2C6H5CH3}, S-shaped for {x1CHCl3+x2CH3COCH3}, {x11,4-C4H8O2+x2(C2H5)3N} and {x1(CH3)2CHOCH(CH3)2+x2(C2H5)3N}, and negative for the other systems.
Similar content being viewed by others
References
E. Wilhelm,Thermochim. Acta 94, 47 (1985).
E. Wilhelm,Thermochim. Acta 162, 43 (1990).
J.-P. E. Grolier and E. Wilhelm,Pure Appl. Chem. 63, 1427 (1991).
E. Wilhelm, A. Inglese, J.-P. E. Grolier, and H. V. Kehiaian,J. Chem. Thermodyn. 14, 33 (1982).
E. Wilhelm, A. Inglese, J.-P. E. Grolier, and H. V. Kehiaian,J. Chem. Thermodyn. 14, 517 (1982).
E. Wilhelm, a. Inglese, A. H. Roux, and J.-P. E. Grolier,Calorim. Anal. Therm. 15, 108 (1984).
a. H. Roux, J.-P. E. Grolier, A. Inglese, and E. Wilhelm,Ber. Bunsenges. Phys. Chem. 88, 986 (1984).
A. Lainez, M. Rodrigo, A. H. Roux, J.-P. E. Grolier, and E. Wilhelm,Calorim. Anal. Therm. 16, 153 (1985).
E. Wilhelm, A. H. Roux, G. Roux-Desgranges, M. Rodrigo, A. Lainez, and J.-P. E. Grolier,Calorim. Anal. Therm. 17, 12 (1986).
E. Wilhelm, A. Lainez, M. M. Rodrigo, A. H. Roux, and J.-P. E. Grolier,Calorim. Anal. Therm. 19, C20.1 (1988).
E. Jimenez, G. Roux-Desgranges, J.-P. E. Grolier, and E. Wilhelm,Thermochim. Acta 151, 99 (1989).
E. Wilhelm, E. Jimenez, G. Roux-Desgranges, and J.-P. E. Grolier,J. Solution Chem. 20, 17 (1991).
J.-P. E. Grolier, G. Roux-Desgranges, M. Berkane, and E. Wilhelm,J. Chem. Thermodyn.23, 421 (1991).
A. Lainez, M. M. Rodrigo, E. Wilhelm, and J.-P. E. Grolier,J. Solution Chem. 21, 49 (1992).
J.-P. E. Grolier, G. Roux-Desganges, M. Berkane, E. Jimenez, and E. Wilhelm,J. Chem. Thermodyn. 25, 41 (1993).
R. D. Nelson, Jr., D. R. Lide, Jr., and A. A. Maryott,Selected Values of Electric Dipole Moments for Molecules in the Gas Phase, NSRDS-NBS 10 (National Bureau of Standards, Washington, D. C. 1967).
A. L. McClellan,Tables of Experimental Dipole Moments, (Freeman, San Francisco and London, 1963).
A. L. McClellan,Tables of Experimental Dipole Moments, Vol, 2, (Rahara Enterprises, El Cerito, California, 1974).
J. A. Riddick, W. B. Bunger, and T. K. Sakano,Organic Solvents. Physical Properties and Methods of Purification, 4th edn., (Wiley, New York, 1986).
L. G. Hepler, Z. S. Kooner, G. Roux-Desgranges, and J.-P. E. Grolier,J. Solution Chem. 14, 576 (1985).
J.-P. E. Grolier, G. Roux-Desgranges, Z. S. Kooner, J. F. Smith, and L. G. Hepler,J. Solution Chem. 16, 745 (1987).
L. Barta, Z. S. Kooner, L. G. Hepler, G. Roux-Desgranges, and J.-P. E. Grolier,Can. J. Chem. 67, 1225 (1989).
L. Barta, Z. S. Kooner, L. G. Hepler, G. Roux-Desgranges, and J.-P. E. Grolier,J. Solution Chem. 18, 663 (1989).
IUPAC,Pure Appl. Chem. 64, 1519 (1992).
J.-P. E. Grolier, E. Wilhelm, and M. H. Hamedi,Ber. Bunsenges. Phys. Chem. 82, 1282 (1978).
G. S. Kell,J. Chem. Eng. Data 20, 97 (1975).
J.-L. Fortier and G. C. Benson,J. Chem. Thermodyn. 8, 411 (1976).
E. Wilhelm, J.-P. E. Grolier, and M. H. Karbalai Ghassemi,Ber. Bunsenges. Phys. Chem. 81, 925 (1977).
R. Tanaka, O. Kiyohara, P. J. D'Arcy, and G. C. Benson,Can. J. Chem. 53, 2262 (1975).
T. M. Letcher and J. W. Bayles,J. Chem. Eng. Data 16, 266 (1971).
J. C. Bonnet and F. P. Pike,J. Chem. Eng. Data 17, 145 (1972).
R. Tanaka and G. C. Benson,J. Chem. Eng. Data 21, 320 (1976).
J. Weclawski,Fluid Phase Equil. 12, 155 (1983).
D. R. Stull,J. Am. Chem. Soc. 59, 2726 (1937).
TRC Thermodynamic Tables: Non Hydrocarbons, (Texas A & M University, College Station, Texas 1986) p. 9410.
K. N. Marsh,J. Chem. Thermodyn. 9, 719 (1977).
J.-P. E. Grolier and E. Wilhelm,Fluid Phase Equil. 6, 283 (1981).
J. C. Hubbard,Phys. Rev. 30, 740 (1910).
L. A. K. Staveley, W. I. Tupman, and K. R. Hart,Trans. Faraday Soc. 51, 323 (1955). See also L. A. K. Staveley, K. R. Hart, and W. I. Tupman,Disc. Faraday Soc. 15, 130 (1953).
A. N. Campbell, E. M. Kartzmark, and R. M. Chatterjee,Can. J. Chem. 44, 1183 (1966).
R. K. Nigam, B. S. Mahl, and P. P. Singh,J. Chem. Thermodyn. 4, 41 (1972).
R. P. Rastogi, J. Nath, and R. R. Misra,J. Chem. Thermodyn. 3, 307 (1971).
A. A. Asfour and F. A. L. Dullien,J. Chem. Eng. Data 26, 312 (1981).
T. J. V. Findlay and R. S. Kenyon,Aust. J. Chem. 22, 865 (1969).
B. R. Sharma and P. P. Singh,J. Chem. Thermodyn. 5, 361 (1973).
K. Quitzsch, H.-P. Prinz, K. Sühnel, V. S. Pham, and G. Geiseler,Z. Phys. Chem. 241, 273 (1969).
R. Gopal, S. Agarwal, and D. K. Agarwal,J. Chem. Thermodyn. 8, 801 (1976).
L. A. Beath, S. P. O'Neill, and A. G. Williamson,J. Chem. Thermodyn. 1, 293 (1969).
J. S. Rowlinson and F. L. Swinton,Liquid and Liquid Mixtures, 3rd edn., (McGraw-Hill, New York, 1982).
E. Wilhelm, A. Lainez, and J.-P. E. Grolier,Fluid Phase Equil. 49, 233 (1989).
M. L. McGlashan and R. P. Rastogi,Trans. Faraday Soc. 54, 496 (1958).
C. J. Creswell and A. L. Allred,J. Phys. Chem. 66, 1469 (1962).
R. W. Kershaw and G. N. Malcolm,Trans. Faraday Soc. 64, 323 (1968).
L. A. Beath and A. G. Williamson,J. Chem. Thermodyn. 1, 51 (1969).
T. J. V. Findlay, J. S. Keniry, A. D. Kidman, and V. A. Pickles,Trans. Faraday Soc. 63, 846 (1967).
B. B. Howard, C. F. Jumper, and M. T. Emerson,J. Molec. Spectrosc. 10, 117 (1963).
M. L. McGlashan, D. Stubley, and H. Watts,J. Chem. Soc. A, 673 (1969).
D. V. Fenby, A. Chand, A. Inglese, J.-P. E. Grolier, and H. V. Kehiaian,Aust. J. Chem. 30, 1401 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grolier, J.P.E., Roux-Desgranges, G., Berkane, M. et al. Heat capacities and densities of mixtures of very polar substances. 3. Mixtures containing either trichloromethane or 1,4-dioxane or diisopropylether. J Solution Chem 23, 153–166 (1994). https://doi.org/10.1007/BF00973543
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00973543