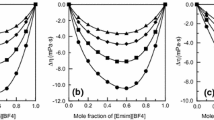
Abstract
The viscosities of most alkali and tetraalkylammonium halides have been measured in water at 25°C. The relative viscosities can be fitted, up to 1M, with the relationη r =1+A η c1/2+B η c+D η 2. TheA η term depends on long-range coulombic forces, andB η is a function of the size and hydration of the solute. When combined with partial-molal-volume data, the difference B η −0.0025V° is mostly a measure of the solute-solvent interactions. IonicB η are obtained if the tetraethylammonium ion is assumed to obey Einstein's law. TheD η parameter depends on higher terms of the long-range coulombic forces, on higher terms of the hydrodynamic effect, and on structural solute-solute interactions. As such, it cannot be interpreted unambiguously.
Similar content being viewed by others
References
R. H. Stokes and R. Mills,Viscosity of Electrolytes and Related Properties (Pergamon Press, Inc., New York, 1965).
M. Kaminsky,Z. Phys. Chem. (Frankfurt) 12, 206 (1957).
D. Feakins and D. G. Lawrence,J. Chem. Soc. (A),212 (1966).
S. Lengyel,J. Chim. Phys. Special Number (October), 28 (1969).
C. T. Robertson and H. J. U. Tyrrell,J. Chem. Soc. (A) 1938 (1969).
J. E. Desnoyers, M. Arel, and P. A. Leduc,Can. J. Chem. 47, 547 (1969).
J. Salvinien, B. Brun, and J. Molenat,J. Chim. Phys. Special Number (October), 19 (1969).
B. E. Conway, R. E. Verrall, and J. E. Desnoyers,Trans. Faraday Soc. 62, 2738 (1966).
J. E. Desnoyers, M. Arel, G. Perron, and C. Jolicoeur,J. Phys. Chem. 73, 3346 (1969).
R. L. Kay, T. Vituccio, C. Zawoyski, and D. F. Evans,J. Phys. Chem. 70, 2336, (1966).
C. Jolicoeur, P. Philip, G. Perron, P. A. Leduc, and J. E. Desnoyers,Can. J. Chem. (in press).
G. Perron and J. E. Desnoyers,J. Chem. Eng. Data 17, 136 (1972).
R. A. Robinson and R. H. Stokes,Electrolyte solutions (Butterworths, London, 1959).
E. R. Nightingale,J. Phys. Chem. 66, 894 (1962).
A. Einstein,Ann. Phys. 19, 289 (1906);34, 591 (1911).
B. E. Conway, J. E. Desnoyers, and R. E. Verrall,J. Phys. Chem. 75, 3031 (1971).
R. L. Kay and D. F. Evans,J. Phys. Chem. 69, 4216 (1965).
C. V. Krisnan and H. L. Friedman,J. Phys. Chem. 74, 2356 (1970).
R. L. Kay,Advan. Chem. Ser. 73, 1 (1968).
C. M. Criss and M. J. Mastroianni,J. Phys. Chem. 75, 2532 (1971).
F. J. Millero,Chem. Rev. 71, 147 (1971).
P. R. Philip and J. E. Desnoyers,J. Solution Chem. (in press).
V. D. Laurence and J. H. Wolfenden,J. Chem. Soc., 1144 (1934).
G. Jones and S. K. Tally,J. Am. Chem. Soc. 55, 1624, (1933).
J. E. Desnoyers, L. Francescon, P. Picker, and C. Jolicoeur,Can. J. Chem. 49, 3460 (1971).
H. Falkenhagen and E. L. Vernon,Z. Physik. 33, 140 (1932).
D. G. Thomas,J. Colloid Sci. 20, 267 (1965).
B. R. Breslau and I. F. Miller,J. Phys. Chem. 74, 1056 (1970).
S. P. Moulik,J. Phys. Chem. 72, 4682 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Desnoyers, J.E., Perron, G. The viscosity of aqueous solutions of alkali and tetraalkylammonium halides at 25°C. J Solution Chem 1, 199–212 (1972). https://doi.org/10.1007/BF00645101
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00645101