Abstract
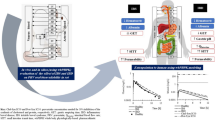
Lumping is a common pragmatic approach aimed at the reduction of whole-body physiologically based pharmacokinetic (PBPK) model dimensionality and complexity. Incorrect lumping is equivalent to model misspecification with all the negative consequences to the subsequent model implementation. Proper lumping should guarantee that no useful information about the kinetics of the underlying processes is lost. To enforce this guarantee, formal standard lumping procedures and techniques need to be defined and implemented. This study examines the lumping process from a system theory point of view, which provides a formal basis for the derivation of principles and standard procedures of lumping. The lumping principle in PBPK modeling is defined as follows: Only tissues with identical model specification, and occupying identical positions in the system structure should be lumped together at each lumping iteration. In order to lump together parallel tissues, they should have similar or close time constants. In order to lump together serial tissues, they should equilibrate very rapidly with one another. The lumping procedure should include the following stages: (i) tissue specification conversion (when tissues with different model specifications are to be lumped together); (ii) classification of the tissues into classes with significantly different kinetics, according to the basic principle of lumping above; (iii) calculation of the parameters of the lumped compartments; (iv) simulation of the lumped system; (v) lumping of the experimental data; and (vi) verification of the lumped model. The use of the lumping principles and procedures to be adopted is illustrated with an example of a commonly implemented whole-body physiologically based pharmacokinetic model structure to characterize the pharmacokinetics of a homologous series of barbiturates in the rat.
Similar content being viewed by others
REFERENCES
S. B. Charnik, R. Kawai, J. R. Nedelman, M. Lemaire, W. Niederberger, and H. Sato. Physiologically based modeling as a tool for drug development. J. Pharmacokin. Biopharm. 23:217–235 (1995).
K. B. Bischoff. Physiological pharmacokinetics. Bull. Math. Biol. 48:309–322 (1986).
M. Rowland. Physiologic pharmacokinetic models and interanimal species scaling. In M. Rowland, and G. T. Tucker (eds.), Pharmacokinetics: Theory and Methodology. International Encyclopedia of Pharmacology and Therapeutics, Section 122, Pergamon, Oxford, 1986, chap. 4, pp. 69–88.
L. E. Gerlovski and R. K. Jain. Physiologically based pharmacokinetic modeling: Principles and applications. J. Pharm. Sci. 72:1003–1129 (1983).
M. E. Andersen. Physiologically based pharmacokinetic (PB-PK) models in the study of the disposition and biological effects of xenobiotics and drugs. Toxicol. Lett. 82/83:341–348 (1995).
A. Bernareggi and M. Rowland. Physiological modeling of Cyclosporine kinetics in rat and man. J. Pharmacokin. Biopharm. 19:21–50 (1991).
R. A. Shipley and R. E. Clark. Tracer Methods for In Vivo Kinetics, Academic Press, New York, 1972.
C. W. Sheppard. Basic principles of the tracer method, Wiley, New York, 1962, p. 64.
W. F. Ebling, D. R. Wada, and D. R. Stanski. From piecewise to full pharmacokinetic modeling: applied to Thiopental disposition in the rat. J. Pharmacokin. Biopharm. 22:259–292 (1994).
S. Bjorkman, D. R. Wada, D. R. Stanski, and W. F. Ebling. Comparative pharmacokinetics of Fentanyl and Alfentanil in rats and humans based on parametric single-tissue models. J. Pharmacokin. Biopharm. 22:381–410 (1994).
R. Kawai, M. Lemaire, J-L. Steimer, A. Bruelisauer, W. Niederberger, and M. Rowland. Physiologically based pharmacokinetic study on a Cyclosporine derivative, SDZ IMM 125. J. Pharmacokin. Biopharm. 22:327–365 (1994).
H. Sato, Y. Sugiyama, Y. Sawada, T. Iga, and M. Nanano. Physiologically based pharmacokinetics of radioiodinated human β-endorphin in rats. Drug Metab. Dispos. 15:540–550 (1987).
A. Tsuji, K. Hiside, N. Minami, E. Nakashima, T. Terasaki, and T. Yamana. Physiologically based pharmacokinetic model for Cefazolin in rabbits and preliminary extrapolation to man. Drug Metab. Dispos. 13:729–739 (1985).
A. Tsuji, H. Sato, I. Tamai, H. Adachi, T. Nishihara, M. Ishiguro, N. Ohnuma, and T. Noguchi. Physiologically based pharmacokinetics of a new penem, SUN5555, for evaluation of in vivo efficacy. Drug Metab. Dispos. 18:245–252 (1990).
L. Dedic and M. Durisova. Frequency response method in pharmacokinetics. J. Pharmacokin Biopharm. 22:293–307 (1994).
J. M. van Rossum, J. E. G. M. de Bie, G. van Lingen, and H. W. A. Teeuwen. Pharmacokinetics from dynamical systems point of view. J. Pharmacokin. Biopharm. 17:365–397 (1989).
K. Godfrey. Compartmental Models and Their Application, Academic Press, 1983.
D. A. Anderson. Compartmental Modeling and Tracer Kinetics. Lecture Notes in Biomathematics, Vol. 50, Springer-Verlag, 1983.
M. Healey. Principles of Automatic Control, Hodder and Stoughton, London, 1975.
O. I. Elgerd. Control System Theory, McGraw-Hill, Tokyo, 1967.
G. J. Murphy. Basic Automatic Control Theory, D. van Nostrand, Princeton, 1957.
I. Nestorov, L. J. Aarons, and M. Rowland. Physiologically based pharmacokinetic modeling of a homologous series of barbiturates in the rat: A sensitivity analysis. J. Pharmacokin. Biopharm. 25:413–447 (1997).
G. Blakey, I. Nestorov, P. Arundel, L. Aarons, and M. Rowland. Quantitative structure—pharmacokinetics relationships: I. Development of a whole-body physiologically based model to characterize changes in pharmacokinetics across a homologous series of barbiturates in the rat. J. Pharmacokin. Biopharm. 25:277–312 (1997).
K. Kakemi, T. Arita, R. Hori, and R. Konishi. Absorption and excretion of drugs XXXII. Absorption of barbituric acid derivatives from rat small intestine. Chem. Pharm Bull. 15:1883–1887 (1967).
B. Davies and T. Morris. Physiological parameters in laboratory animals and humans. Pharma. Res. 10:1093–1095 (1993).
M. Rowland and T. N. Tozer. Clinical Pharmacokinetics: Concepts and Applications, 3rd ed. Lea and Febiger, Philiadelphia, 1995.
ACSL Reference Manual, Version 11, MGA Software, Concord, MA 01742, 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nestorov, I.A., Aarons, L.J., Arundel, P.A. et al. Lumping of Whole-Body Physiologically Based Pharmacokinetic Models. J Pharmacokinet Pharmacodyn 26, 21–46 (1998). https://doi.org/10.1023/A:1023272707390
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1023272707390