Abstract
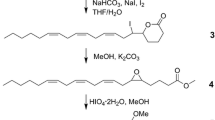
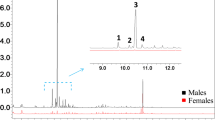
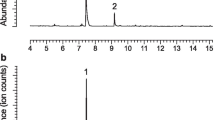
Syntheses of all four Stereoisomers (2S,5S; 2S,5R;2R,5R; and2R,5S) of chalcogran, a major component of the aggregation pheromone ofPityogenes chalcographus, and of all four isomers (2Z,4Z; 2Z,4E; 2E,4E; and 2E,4Z) of methyl 2,4-decadienoate (MD), the second major pheromone component, are briefly described. Attraction responses of walking beetles of both sexes were tested to mixtures of the synergistic pheromone components or analogs. These bioassays showed that theE,Z isomer of MD is the most active when tested with chalcogran. When tested with (E,Z)-MD, (2S,5R)-chalcogran was the most active stereoisomer, while 2R,5R and 2R,5S isomers had intermediate activities, and the 2S,5S isomer was inactive. There was no evidence that the relatively less active Stereoisomers of chalcogran inhibited or promoted attraction to (2S,5R)-chalcogran with (E,Z)-MD. Male beetles only produce the activeE,Z isomer of MD (inactive alone) and their hindguts contain the most active (2S,5R)- and least active (2S,5S)-chalcogran. A mixture of all MD isomers with racemic chalcogran was not significantly different in attractivity compared to (E,Z)-MD with racemic chalcogran, indicating no synergistic or inhibitory effects of the inactive isomers of MD.
Similar content being viewed by others
References
Baeckström, P., Jacobsson, U., Norin, T. andUnelius, C.R. 1988. Synthesis and characterization of all four isomers of methyl 2,4-decadienoate for an investigation of the pheromone components ofPityogenes chalcographus.Tetrahedron 44:2541–2548.
Banerji, A., andPal, S.C. 1983. Total synthesis of sylvamide, aPiper alkamide.Phytochemistry 22:1028–1030.
Björkling, F., Norin, T., andUnelius, C.R. 1987. A stereospecific synthesis of all fourisomers of 9,11-tetradecadienyl acetate using a general method applicable to 1,3-dienes.J. Org. Chem. 52:292–294.
Borden, J.H., Chong, L., Mclean, J.A., Slessor, K.M., andMori, K. 1976.Gnathotrichus sulcatus: Synergistic response to enantiomers of the aggregation pheromone sulcatol.Science 192:894–896.
Browne, L.E., Birch, M.C., andWood, D.L. 1974. Novel trapping and delivery systems for airborne insect pheromones.J. Insect Physiol. 20:183–193.
Byers, J.A., andWood, D.L. 1981. Interspecific effects of pheromones on the attraction of the bark beetles,Dendroctonus brevicomis andIps paraconfusus in the laboratory.J. Chem. Ecol. 7:9–18.
Byers, J.A., Lanne, B.S., Löfqvist, J., Schlyter, F., andBergström, G. 1985. Olfactory recognition of host-tree susceptibility by pine shoot beetles.Naturwissenschaften 72:324–326.
Byers, J.A., Birgersson, G., Löfqvist, J., andBergström, G. 1988. Synergistic pheromones and monoterpenes enable aggregation and host recognition by a bark beetle.Naturwissenschaften 75:153–155.
Byers, J.A.,Birgersson, G.,Löfqvist, J.,Appelgren, M., andBergström, G. 1989. Isolation of pheromone synergists ofPityogenes chalcographus from complex insect-plant odors by gas Chromatographie fractionation and subtractive-combination bioassay.J. Chem. Ecol. (submitted).
Cardé, R.T., andBaker, T.C. 1984. Sexual communication with pheromones. pp. 355–383,in W.J. Bell and R.T. Cardé (eds.). Chemical Ecology of Insects. Chapman and Hall, London.
Crombie, L., andDenman, R. 1984. Insecticidal amides. Synthesis of natural 2(E),4(E),10(E)-pipercide, its 2(E),4(E),10(Z)-stereomer, and related isobutylamides.Tetrahedron Lett. 25:4267–4270.
Deans, D.R. 1981. Use of heart cutting in gas chromatography: A review.J. Chromatogr. 203:19–28.
Fieser, L.F. 1964.Organic Experiments. p. 162 D.C. Heath & Co., Boston.
Francke, W., Heemann, V., Gerken, B., Renwick, J.A.A., andVitë, J.P. 1977. 2-Ethyl-1,6-dioxaspiro[4.4]nonane, principal aggregation pheromone ofPityogenes chalcographus (L.).Naturwissenschaften 64:590–591.
Francke, W., Reith, W., andSinnwell, V. 1980. Bestimmung der relativen Konfiguration bei Spiroacetalen durch1H-und13C-NMR-Spektroskopie.Chem. Ber. 113:2686–2693.
Garigipati, R.S., andWeinreb, S.M. 1983. Stereospecific synthesis of acyclic unsaturated amino alcohols. A new approach to threo- and erythro-sphingosine.J. Am. Chem. Soc. 105:4499–4501.
Högberg, H.E., Hedenström, E., Isaksson, R., andWassgren, A.B. 1987. Preparation of the four stereoisomers of chalcogran, pheromone components ofPityogenes chalcographus and of both enantiomers of γ-caprolactone, pheromone component ofTrogoderma granarium.Acta. Chem. Scand. Ser. B 41:694–697.
Isaksson, R., andRöschester, J. 1985. Preparative and analytical enantiomer separation of some delta-1,3-thiazoline-2-thiones on swollen microcrystalline triacetylcellulose (TAC).J. Org. Chem. 50:2519–2521.
Koppenhoefer, B., Hintzer, K., Weber, R., andSchurig, V. 1980. Quantitative Trennung der Enantiomerenpaare des Pheromons 2-Ethyl-1,6-dioxaspiro[4.4]nonan durch Komplexierung-schromatographie an einem optisch aktiven Metallkomplex.Angew. Chem. 92:473–474.
Leadbetter, G., andFlimmer, J.R. 1979. An improved preparation of some insect sex attractants: Synthesis and separation of geometrical isomers by formation of urea complexes.J. Chem. Ecol. 5:101–108.
Pearce, G.T., Gore, W.E., Silverstein, R.M., Peacock, J.W., Cuthbert, R.A., Lanier, G.N., andSimeons, J.B. 1975. Chemical attractants for the smaller European elm bark beetle,Scolytus multistriatus (Coleoptera: Scolytidae).J. Chem. Ecol. 1:115–124.
Renwick, J.A.A., Hughes, P.R., andKrull, I.S. 1976. Selective production ofcis- andtrans- verbenol from (+)-α-pinene by a bark beetle.Science 191:199–201.
Rickards, G., andWeiler, L. 1978. Stereoselective synthesis of 1-substituted (E,E)- and (E,Z)-2,4-decadienyl derivatives.J. Org. Chem. 43:3607–3609.
Roush, W.R. 1980. Total synthesis of (±)-dendrobine.J. Am. Chem. Soc. 102:1390–1404.
Schurig, V., andWeber, R. 1984. Use of glass and fused-silica open tubular columns for the separation of structural, configurational and optical isomers by selective compiexation gas chromatography.J. Chromatogr. 289:321–332.
Silverstein, R.M., Rodin, J.O., andWood, D.L. 1966. Sex attractants in frass produced by maleIps paraconfusus in ponderosa pine.Science 154:509–510.
Silverstein, R.M., Rodin, J.O., andWood, D.L. 1967. Methodology for isolation and identification of insect pheromones with reference to studies on California five-spinedIps.J. Econ. Entomol. 60:944–949.
Silverstein, R.M., Brownlee, R.G., Bellas, T.E., Wood, D.L., andBrowne, L.E. 1968. Brevicomin: Principal sex attractant in the frass of the female western pine beetle.Science 59:889–890.
Smith, L.R.,Williams, H.J., andSilverstein, R.M. 1978. Facile synthesis of optically active 2-ethyl-1,6-dioxaspiro[4.4]nonane, component of the aggregation pheromone of the beetlePityogenes chalcographus (L.).Tetrahedron Lett. 3231–3232.
Stille, J.K., andGroh, B.L. 1987. Stereospecific cross-coupling of vinyl halides with vinyl tin reagents catalyzed by palladium.J. Am. Chem. Soc. 109-813-817.
Vité, J.P. 1965. 1st die vorbeugende Begiftung von Fangbaume zweckmassig?Allg. Forstzeitschrift 20:438–439.
Vité, J.P., andRenwick, J.A.A. 1970. Differential diagnosis and isolation of population attractants.Contrib. Boyce Thompson Inst. 24:323–328.
Weir, J.R., Patel, B.A., andHeck, R.F. 1980. Palladium-catalyzed triethylammonium formate reductions. 4. Reduction of acetylenes tocis monoenes and hydrogenolysis of tertiary allylic amines.J. Org. Chem. 45:4926–4931.
Wood, D.L. 1982. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles.Annu. Rev. Entomol. 27:411–446.
Wood, D.L., Browne, L.E., Ewing, B., Lindahl, K., Bedard, W.D., Tilden, P.E., Mori, K., Pitman, G.B., andHughes, P.R. 1976. Western pine beetle: Specificity among enantiomers of male and female components of an attractive pheromone.Science 192:896–898.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byers, J.A., Högberg, HE., Unelius, C.R. et al. Structure-activity studies on aggregation pheromone components ofPityogenes chalcographus (Coleoptera: Scolytidae). J Chem Ecol 15, 685–695 (1989). https://doi.org/10.1007/BF01014711
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01014711