Abstract
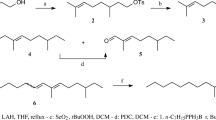
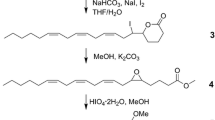
A new efficient stereoselective synthesis for (Z,E)-3,5-tetradecadienyl acetate (Z3,E5–14∶Ac) a potent male sex attractant for the carpenterworm,Prionoxystus robiniae (Peck), was developed. The effects of the other three isomers (Z,Z; E,Z; E,E) on the field attractiveness of theZ,E isomer toward maleP. robiniae were determined. TheZ, Z isomer inhibited attraction, theE, E isomer synergized attraction, and theE,Z isomer had no effect on attraction. Seven monounsaturated 14-carbon acetates were evaluated for their effect on the attractiveness ofZ3,E5–14∶Ac. (E)-3-Tetradecenyl acetate enhanced attraction and (Z)-9- and (E)-5-tetradecenyl acetate reduced trap captures. (Z,E)-3,5-Tetradecadien-1-ol also reduced the attractiveness ofZ3,E5–14∶Ac.
Similar content being viewed by others
References
Brandsma, L. 1971. Preparative Acetylenic Chemistry, pp. 98–99. Elsevier Publishing, New York.
Chodkiewicz, W.Y., andCadiot, P. 1955. Nouvelle synthèse de composés polyacétyléniques conjugués symétriques et dissymétriques.Compt. Rend. 241:1055–1057.
Doolittle, R.E., Roelofs, W.L., Solomon, J.D., Cardé, R.T., andBeroza, M. 1976a. (Z,E)-3,5-Tetradecadien-1-ol acetate sex attractant for the carpenterworm moth,Prionoxystus robiniae (Peck) (Lepidoptera: Cossidae).J. Chem. Ecol. 2:399–410.
Doolittle, R.E., Tagestad, A., andMcKnight, M.E. 1976b. Trapping carpenterworms and aspen carpenterworms with sex attractants in North Dakota.Environ. Entomol. 5:267–269.
Heath, R.R., andDoolittle, R.E. 1983. Derivatives of cholesterol cinnamate: A comparison of the separations of geometrical isomers when used as gas Chromatographic stationary phases.J. High Resolut. Chromatogr. Chromatogr. Commun. 6:16–19.
Heath, R.R., Tumlinson, J.H., andDoolittle, R.E. 1977. Analytical and preparative separation of geometrical isomers by high efficiency silver nitrate liquid chromatography.J. Chromatogr. Sci. 15:10–13.
Henrick, C.A. 1978. The synthesis of insect sex pheromones.Tetrahedron 33:1–48.
Klun, J.A., Flimmer, J.R., andBierl-Leonhardt, B.A. 1979. Trace chemicals: The essence of sexual communication systems inHeliothis species.Science 204:1328–1330.
Miyashita, N., Yoshikoshi, A., andGrieco, P.A. 1977. Pyridiniump-toluenesulfonate. A mild and efficient catalyst for tetrahydropyranylation of alcohols.J. Org. Chem. 42:3772–3774.
Mori, K. 1979. Synthetic chemistry of insect pheromones and juvenile hormones, pp. 11–209,in R. Bognár, V. Buckner, Cs. Szantay (eds.). Recent Developments in the Chemistry of Natural Carbon Compounds, Vol. 9, Akademiae Kiado, Budapest.
Mori, K. 1981. The synthesis of insect pheromones, pp. 1–183,in J.W. ApSimon (ed.). Total Synthesis of Natural Products, Vol. 4, John Wiley & Sons, New York.
Rossi, R. 1977. Insect pheromones; 1. Synthesis of achiral components of insect pheromones.Synthesis 817–836.
Solomon, J.D., andMorris, R.C. 1966. Sex attraction of the carpenterworm moth.J. Econ. Entomol. 59:1534–1535.
Solomon, J.D., Doolittle, R.E., andBeroza, M. 1972. Isolation and analysis of the carpenterworm sex pheromone.Ann. Entomol. Soc. Am. 65:1058–1061.
Solomon, J.D., Dix, M.E., andDoolittle, R.E. 1978. Attractiveness of the synthetic carpenterworm sex attractant increased by isomeric mixtures and prolonged by preservatives.Environ. Entomol. 7:39–41.
Sonnet, P.E. 1984. Tabulations of selected methods of synthesis that are frequently employed for insect sex pheromones, emphasizing the literature of 1977–1982, pp. 371–403,in H. Hummel (ed.). Insect Pheromones. Springer Verlag, New York.
Steck, W., Underhill, E.W., andChisholm, M.D. 1980. Trace components in lepidopterous sex attractants.Environ. Entomol. 9:583–585.
Steck, W., Underhill, E.W., andChisholm, M.D. 1982. Structure-activity relationships in sex attractants for North American noctuid moths.J. Chem. Ecol. 8:731–754.
Steel, R.G.D., andTorrie, J.H. 1960. Principles and Procedures of Statistics. McGraw-Hill, New York, 481 pp.
Tumlinson, J.H., Mitchell, E.R., Browner, S.M., Mayer, M.S., Green, N., Hines, R., andLindquist, D.A. 1972.cis-7-Dodecen-1-ol, a potent inhibitor of the cabbage looper sex pheromone.Environ. Entomol. 1:354–358.
Zweifel, G., andBacklund, S.J. 1978. Synthesis of 1,4-disubstituted (E,Z)-1,3-dienes from lithium dicyclohexyl (trans-1-alkenyl) (1-alkynyl) borates.J. Organomet. Chem. 156:159–170.
Zweifel, G., andPolston, N.L. 1970. Selective hydroboration of conjugated diynes with dialkylboranes. A convenient route to conjugatedcis-enzymes, α,β-acetylenic ketones andcis,cis-dienes.J. Am. Chem. Soc. 92:4068–4071.
Author information
Authors and Affiliations
Additional information
Mention of a commercial or properietary product does not constitute an endorsement by the USDA.
Rights and permissions
About this article
Cite this article
Doolittle, R.E., Solomon, J.D. Stereoselective synthesis of (Z,E)-3,5-tetradecadienyl acetate: Sex attractant for carpenterworm moth,Prionoxystus robiniae (Peck) (Lepidoptera: Cossidae) and effect of isomers and monounsaturated acetates on its attractiveness. J Chem Ecol 12, 619–633 (1986). https://doi.org/10.1007/BF01012097
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01012097