Abstract
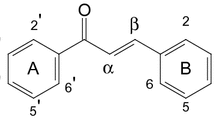
Wax (mp 102–103 °C) taken from the cochineal insectDactylopius confusus was saponified to give 11-oxotriacontanoic acid (1) and 15-oxotetratriacontanol (3) in good yields. The structure of these hydrolysis products follows most directly from examination of the mass spectra of the corresponding methyl ester (2) and acetate (4). These spectra are dominated by McLafferty rearrangements initiated by the ketonic carbonyl groups, and resulting in preferential loss of the unfunctionalized hydrocarbon end of the long-chain esters. The structures are confirmed by synthesis of both hydrolysis products via reaction of di-n-nonadecyl cadmium with the appropriate acid chlorides and of the C64 wax ester (5) itself by acid-catalyzed condensation of these moieties. Mass spectral examination of the cochineal wax previously characterized as 15-oxotetratriacontanyl 13-oxodotriacontanoate on the basis of purely chemical evidence confirms the assignment, and the Wooly Alder AphidProciphilus tessalatus is now found to produce the same C66 keto-ester.
Similar content being viewed by others
References
Cason, J. 1947. The use of organocadmium reagents for the preparation of ketones.Chem. Rev. 40:15–32.
Cason, J., Wolfhagen, H.J., Tarpey, N., andAdams, R.E. 1949. Branched-chain fatty acids. X. Synthesis of acids with branching methyl groups near the carboxyl.J. Org. Chem. 14:147–154.
Chibnall, A.C., Latner, A.L., Williams, E.J., andAyre, C.A. 1934. Constitution of coccerin.Biochem. J. 28:313–325.
Chutt, P. 1926. Préparation d'acides polyméthylène-dicarboniques de 11 à 19 atomes de carbone et de quelques-uns de leur derivés.Helv. Chim. Acta 9:264–278.
Chutt, P., andHausser, J. 1929. Sur les acides-alcools polyméthylène-carboniques de 8 à 21 atomes de carbone.Helv. Chim. Acta 12:463–492.
Ferris, G.F. 1955.Atlas of the Scale Insects of North America, Volume VII. Stanford University Press, Stanford, California. 233 pp.
Jones, R.G. 1947. The synthesis of some long chain primary alcohols and related compounds.J. Am. Chem. Soc. 69:2350–2354.
Ryhage, R., andStenhagen, E. 1960. Mass spectrometric studies. VI. Methyl esters of normal chain oxo-, hydroxy-, methoxy-, and epoxy-acids.Arkiv. Kemi 15:545–574.
Ryhage, R., andStenhagen, E. 1963.Mass Spectrometry of Organic Ions. Academic Press, London, pp. 430–444.
Takemoto, T., andKondo, Y. 1964. A keto acid extracted from the seeds ofPapaver somniferum.Yakugaku Zasshi 84:474–477.
Tulloch, A.P. 1970. Composition of beeswax and other waxes secreted by insects.Lipids 5:247–258.
Author information
Authors and Affiliations
Additional information
Paper No. 40 of the seriesDefense Mechanisms of Arthropods.
Rights and permissions
About this article
Cite this article
Meinwald, J., Smolanoff, J., Chibnall, A.C. et al. Characterization and synthesis of waxes from homopterous insects. J Chem Ecol 1, 269–274 (1975). https://doi.org/10.1007/BF00987875
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00987875