Abstract
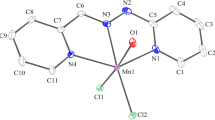
Naphthoquinonedithiolate (nqdt) is a “noninnocent” ligand forming dithiolene transition metal complexes. Most [M(nqdt)2]−1 and [M(nqdt)2]−2 complex ions are planar with each forming an isomorphous series with the (n-butyl)4N+ (tba) counter ion. The cobalt complex is isolated as a step dimer (tba)2[Co(nqdt)2]2 where two square-planar monomer units are bonded via Co−S intermolecular interactions.Ab initio andsemi-empirical calculations are used to investigate the complex ions. The complexes form tba single crystals only with difficulty, and an extensive study with ions of various shapes and charges failed to identify a more satisfactory counter ion. The magnetic susceptibilities of four complexes are discussed. The (tba)[Ni(nqdt)2] complex undergoes a weak ferromagnetic transition at 4.43 K while a mixed oxidation state Mn(II) complex exhibits a weak ferromagnetic transition at 40K. The crystal structures of (tba) [Cu(nqdt)2], (tba)[Ni(nqdt)2], (tba)2[Ni(nqdt)2], (tba)2[Pd(nqdt)2], {(tba)[Co(nqdt)2]}2 and four side reaction products 5,7,12,14-tetrahydrodibenzo-[b,i]thianthrene-5,7,12,14-tetraone (2), benzocyclohexa-2,5-diene-1,4-dione-1,3-thiole-2-thione (3), a thiomethyl derivative (4), and an unusual oxidatively coupled product (5) are reported.
Similar content being viewed by others
References
Schrauzer, G.N.Acc. Chem. Res. 1969,2, 72.
Schrauzer, G.N.Transit. Metal Chem. 1968,4, 299.
McCleverty, J.A.Prog. Inorg. Chem. 1968,10, 49.
Davison, A.; Edelstein, N.; Holm, R.H.; Maki, A.H.Inorg. Chem. 1963,10, 1227.
Schrauzer, G.N.; Mayweg, V.P.J. Am. Chem. Soc. 1965,87, 1483.
Schrauzer, G.N.; Mayweg, V.P.J. Am. Chem. Soc. 1965,87, 3585.
Schmit, R.; Maki, A.H.:J. Am. Chem. Soc. 1968,90, 2288.
Watson, W.H.; Eduok, E.E.; Kashyap, R.P.; Krawiec, M.Tetrahedron 1993,49, 3035.
Sheldrick, G.M.SHELXS86. Program for the Solution of Crystal Structures; Univ. of Gottingen: Germany,1986.
TEXSAN-TEXRAYStructure Analysis Package; Molecular Structure Corporation,1985.
Spek, A.L.Acta Crystallogr. 1990,A46, c34.
O'Conner, C.J.Prog. Inorg. Chem. 1982,29, 203.
Spartanversion 4.0, Wavefunction. Inc. 18401 Von Karman Ave., #370 Irvine, CA 92715.
Gaussian 94 (version 2.1), Frisch, M.J.; Trucks, F.W.; Schlegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Robb, M.A.; Cheeseman, J.R.; Keith, T.A.; Petersson, G.A.; Montgomery, J.A.; Raghavachari, K.; Al-Laham, M.A.; Zakrzewski, V.G.; Ortiz, J.V.; Foresman, J.B.; Cioslowski, J.; Stefanov, B.B.; Nanayakkara, A.; Challacombe, M.; Peng, C.Y.; Ayala, P.Y.; Chen, M.; Wong, M.W.; Andres, J.L.; Replogle, E.S.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Binkley, J.S.; Defrees, D.J.; Baker, J.; Stewart, J.P.; Head-Gordon, M.; Gonzalez, C.; Pople, J.A. Gaussian, Inc., Pittsburgh PA,1995.
Davisson, A.; Edelstein, N.; Holm, R.H.; Maki, A.H.Inorg. Chem.,1964,3, 814.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eduok, E.E., Krawiec, m., Buisson, YS.L. et al. Structure and properties of transition metal complexes of naphthoquinonedithiolate. J Chem Crystallogr 26, 621–638 (1996). https://doi.org/10.1007/BF01668623
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01668623