Summary
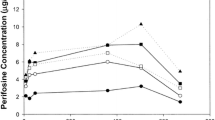
Tiazofurin, an investigational antimetabolite, is undergoing clinical evaluation in leukemia. We analyzed the data base of 198 patients entered in Phase I trials to characterize the incidence and severity of toxicities associated with tiazofurin according to dose and schedule. Severe myelosuppression occurred infrequently, and was not dose-dependent. A five day bolus schedule had a higher incidence of severe or life-threatening neutropenia than other schedules. Tiazofurin produced lymphopenia which was not dose-dependent in the range of 23–36% decrease from baseline, and the effect on lymphocyte count was generally greater than the decline in neutrophil count. Non-hematologic toxicity of a moderate or worse severity (≥ grade 2) included nausea and vomiting (18% of all courses), serum transaminase elevations (SGOT, 16%; SGPT, 9%), rash (9%), stomatitis (3%), conjunctivitis (3%), headache (10%), other signs of central nervous system toxicity (8%), and cardiac toxicity, primarily pleuropericarditis (4%). Dose-related cutaneous toxicity, headache, and nausea and vomiting were evident in the five day bolus schedule, and myalgia was more frequently reported at higher doses on the single dose schedule. The five day continuous infusion (CI) schedule had a higher incidence of neurotoxicity, cardiac toxicity, SGPT elevations and ocular toxicity than the daily for five days bolus schedule, but none of these differences attained statistical significance. Although the peak plasma concentrations of tiazofurin achieved with the five day bolus schedule were 3-fold higher than the steady-state plasma levels seen with an equal dose given by CI, the area under the concentration-time curve (AUC) was approximately 1.6-fold higher with CI. These observations suggest that both high peak plasma concentrations (above 400 uM) and prolonged exposure to plasma levels exceeding 50 uM may result in a higher incidence of serious non-hematologic toxicity.
Similar content being viewed by others
References
O'Dwyer PJ, Shoemaker DD, Jayaram HN, Johns DG, Cooney DA, Marsoni S, Malspeis L, Plowman J, Davignon JP, Davis RD: Tiazofurin: A new antitumor agent. Invest New Drugs 2: 79–84, 1984
Jayaram HN, Smith AL, Glazer RI, Johns DG, Cooney DA: Studies on the mechanism of action of 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193)-II. Biochem Pharmacol 31: 3869–3845, 1982
Cooney DA, Jayaram HN, Glazer RI et al.: Studies on the mechanism of action of tiazofurin metabolism to an analog of NAD with potent IMP-dehydrogenase-inhibitory activity. Adv Enz Regul 21: 272–303, 1983
Weber G, Natsumeda Y, Pillwein K: Targets and markers of selective action of tiazofurin. Adv Enz Regul 24: 45–65, 1985
Lee HJ, Pawlak K, Nguyen BT, Robins RK, Sadee W: Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. Cancer Res 45: 5512–5520, 1985
Monks A, Marquez VE, Mao DT, Cysyk RL: Uptake of 2-beta-D-ribofuranosylthiazole-4-carboxamide (tiazofurin) and analogues by the facilitated transport mechanism of erythrocytes. Cancer Lett 28: 1–8, 1985
Boritzki TJ, Berry DA, Besserer JA, Cook PD, Fry DW, Leopold WR, Jackson RC: Biochemical and antitumor activity of tiazofurin and its selenium analog (2-beta-D-ribofuranosyl-4-selenacarboxamide). Biochem Pharmacol 34: 1109–1114, 1985
Berger NA, Berger SJ, Catino DM, Petzold SJ, Robins RK: Modulation of nicotinamide adenine dinucleotide and poly (adenosine diphosphoribose) metabolism by the synthetic “C” nucleoside analogs, tiazofurin and selenazofurin. A new strategy for cancer chemotherapy. J Clin Invest 75: 702–709, 1985
Miller BR, Affigne SM: Purine nucleoside phosphorylase and adenosine deaminase activity in the spleens of C57BL/6 mice bearing Lewis lung carcinoma — effect of tiazofurin. Proc Am Assoc Cancer Res 27: 4, 1986 (Abstr)
Jayaram HN, Pillwein K, Nichols CR, Hoffman R, Weber G: Selective sensitivity to tiazofurin of human leukemic cells. Biochem Pharmacol 35: 2029–2032, 1986
Ahluwalia GS, Jayaram HN, Coony DA: Metabolites of tiazofurin as mediators of its biochemical and pharmacologic effects. Cancer Treat Res 36: 63–102, 1987
Yamada Y, Natsumeda Y, Weber G: Action of the active metabolites of tiazofurin and ribavirin on purified IMP dehydrogenase. Biochemistry 27: 2193–2196, 1988
Jayaram HN: Biochemical mechanisms of resistance to tiazofurin. Adv Enz Regul 24: 67–89, 1986
Knight RD, Mangum J, Lucas DL, Cooney DA, Khan EC, Wright DG: Inosine monophosphate dehydrogenase and myeloid cell maturation. Blood 69: 634–639, 1987
Neidhart J, Metz E, Gochnour D, Dallaire B, Grillo-Lopez A, Malspeis L: Phase I study of tiazofurin (2-beta-D-ribofuranosyl-4-thiazole-carboxamide; NSC 286193). Proc Am Soc Clin Oncol 3: 36, 1984 (Abstr)
Balis FM, Lange BJ, Packer RJ, Holcenberg JS, Ettinger LJ, Sallan SE, Heideman RL, Zimm S, Smithson WA, Cogliano-Shutta NA et al.: Pediatric phase I trial and pharmacokinetic study of tiazofurin (NSC 286193). Cancer Res45: 5169–5172, 1985
Maroun JA, Green R, Stewart DJ: Phase I study of tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide). Proc Am Soc Clin Oncol 4: 48, 1985 (Abstr)
Melink TJ, Von Hoff DD, Kuhn JG, Hersh MR, Sternson LA, Patten TF, Siegler R, Boldt DH, Clark GM: Phase I evaluation and pharmacokinetics of tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide, NSC 286193). Cancer Res 45: 2859–2865, 1985
Currie VE, Budman D, Hancock C, Lokos G, Williams L, Bauer T, Parente R, Young C: Phase I and clinical pharmacologic evaluation of tiazofurin by an intermittent schedule. Proc Am Assoc Cancer Res 26: 188, 1985 (Abstr)
Green RM, Stewart DJ, Maroun JA: Clinical pharmacology of tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide, NSC 286193). Invest New Drugs 4: 387–394, 1986
Melink T, Von Hoff D, Phillips J, Sarosy G, Grever M, Jayaram H, Whitecar J: Phase I trial and biochemical evaluation of tiazofurin on a weekly schedule. Proc Am Assoc Cancer Res 27: 173, 1986 (Abstr)
Roberts JD, Stewart JA, McCormack JJ, Krakoff IR, Culham CA, Hartshorn JN, Newman RA, Haugh LD, Young JA: Phase I trial of tiazofurin administered by i.v. bolus daily for 5 days, with pharmacokinetic evaluation. Cancer Treat Rep 71: 141–149, 1987
Trump DL, Tutsch KD, Koeller JM, Tormey DC: Phase I clinical study with pharmacokinetic analysis of 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193) administered as a five-day infusion. Cancer Res 45: 2853–2858, 1985
Batist G, Klecker RW Jr, Jauaram HN, Jenkins JF, Grygiel J, Ihde DC, Eddy JL, Fine RL, Kerr IG, Collins JM: Phase I and pharmacokinetic study of tiazofurin (NSC 286193) administered by continuous infusion. Invest New Drugs 3: 349–355, 1985
Raghaven D, Bishop J, Sampson D, Grygiel J, Woods R, Coates A, Fox R: Phase I and pharmacokinetic study of tiazofurin (NSC 286193) administered by 5-day continuous infusion. Cancer Chemother Pharmacol 16: 160–164, 1986
Goldberg R, Ahlgren J: Treatment of metastatic malignant melanoma with tiazofurin: A phase II study. Proc Am Soc Clin Oncol 7: 253, 1988 (Abstr)
Dimery IW, Neidhart JA, McCarthy K, Krakoff IH, Hong WK: Phase II trial of tiazofurin in recurrent squamous cell carcinoma of the head and neck. Cancer Treat Rep 71: 425–426, 1987
Maroun JA, Eisenhauer E, Cripps C, Maksymiuk A: Phase II study of tiazofurin in colorectal cancer: A NationalCancer Institute of Canada study. Cancer Treat Rep 71: 1297–1298, 1987
Holoye PY, Carr DT, Dhingra HM, Glisson BS, Lee JS, Murphy WK, Umsawasdi T, Jeffries D: Phase II study of tiazofurin (286193) in the treatment of advanced small cell bronchogenic carcinoma. Invest New Drugs 6: 217–218, 1988
Tricot GJ, Jayaram HN, Nichols CR, Pennington K, Lapis E, Weber G, Hoffman R: Hematological and biochemical action of tiazofurin (NSC 286193) in a case of refractory acute myeloid leukemia. Cancer Res 47: 4988–4991, 1987
Tricot GJ, Jayaram HN, Weber G, Hoffman R: Tiazofurin is a potent antiproliferative agent and induces differentiation in patients with leukemia. Proc Am Assoc Cancer Res 29: 210, 1988 (Abstr)
Weber G, Jayaram HN, Lapis E, Natsumeda Y, Yamada Y, Yamaju Y, Tricot GJ, Hoffman R: Enzyme-patterntargeted chemotherapy with tiazofurin and allopurinol in human leukemia. Adv Enz Regul 27: 405–433, 1988
Fleiss JL: Statistical methods for rates and proportions. John Wiley and Sons, New York, 1981
National Cancer Institute, Division of Cancer Treatment, Clinical Brochure: Tiazofurin (NSC 286193), Bethesda, MD, 1982
Balducci L, Thigpen JT, Hardy C: Hemopoietic effects of tiazofurin in normal and tumor bearing animals. Proc Am Assoc Cancer Res 27: 307, 1986 (Abstr)
Grygiel JJ, Balis FM, Collins JM, Lester CM, Poplack DG: Pharmacokinetics of tiazofurin in the plasma and cerebrospinal fluid of rehesus monkeys. Cancer Res 45: 2037–2039, 1985
Green RM, Hugenholtz H, Richard M, Dennery J, Hopkins H, Thibault M, Stewart DJ: Human central nervous system pharmacology of tiazofurin. J Neurooncol 2: 288, 1984
Arnold ST, Jayaram HN, Harper GR, Litterst CL, Malspies L, DeSouza JV, Staubus AE, Ahluwalia GS, Wison YA, Cooney DA, Johns DG: The disposition and metabolism of tiazofurin in rodents, rabbits and dogs. Drug Metab Dispos 12: 165–173, 1984
Lathan B, Von Hoff DD, Melink TJ, Kisner DL: Screening of Phase I drugs in the human tumor cloning system to pinpoint areas of emphasis in phase II studies: Human Tumor Cloning. Fourth Conference on Human Tumor Cloning. Salmon SE, Trent JM (eds) Orlando, Florida, Grune and Stratton, 1984, p 669
Melink TJ, Von Hoff DD, Clark GM, Coltman CA Jr: Antitumor activity of tiazofurin in a human tumor cloning system. Proc Am Assoc Cancer Res 25: 375, 1984 (Abstr)
Carney DN, Ahluwalia GS, Jayaram HN, Coomey DA, Johns DG: Relationship between the cytotoxicity of tiazofurin and its metabolism by cultured human lung cancer cells. Clin Invest 75: 175–182, 1985
Locker G, Khandekar J, Knop R, Kram J, Carro G, Block T, French S, Tuteur D: Illinois cancer council phase I trial of weekly intravenous aminothiadiazole. Proc Am Soc Clin Oncol 7: 57, 1988 (Abstr)
Stoeckler JD: Purine nucleoside phosphorylase: A target for chemotherapy. In: Glazer R (ed) Recent Developments in Cancer Chemotherapy. CRS Press, Boca Raton, FL, 1984 pp 35–60
Hoffbrand AV, Ma DD, Webster AD: Enzyme patterns in normal lymphocyte subpopulations, lymphoid leukaemias and immunodeficiency syndromes. Clin Haematol 11: 719–741, 1982
Begleiter A, Glazer RI, Israels LG, Pugh L, Johnston JB: Induction of DNA strand breaks in chronic lymphocytic leukemia following treatment with 2′deoxycoformycin in vivo and in vitro. Cancer Res 47: 2498–2503, 1987
Brager PM, Grever MR: 9-beta-D-arabinofuranosyl-2-fluo-roadenine reduces NAD in normal lymphocytes and neoplastic cells in CLL. Proc Am Assoc Cancer Res 27: 21, 1986 (Abstr)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grem, J.L., Rubinstein, L., King, S.A. et al. Clinical toxicity associated with tiazofurin. Invest New Drugs 8, 227–238 (1990). https://doi.org/10.1007/BF00177266
Issue Date:
DOI: https://doi.org/10.1007/BF00177266