Abstract
1. The individual and sequential influence of protein kinase C (PKC), protein kinase A (PKA) and mitogen-activated protein kinase (MAP kinase) on human brain tau was examined.
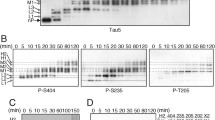
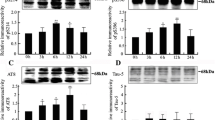
2. A range of PKC concentrations generated certain phosphoepitopes common with paired helical filaments. These epitopes were masked by higher PKC concentrations, suggesting the presence of multiple tau phosphorylation sites for which PKC exhibited differing affinities and/or conformational alterations in tau induced by sequential PKC-mediated phosphorylation.
3. Prior phosphorylation by PKC enhanced the nature and extent of AD-like tau antigenicity generated by subsequent incubation with MAP kinase yet inhibited that generated by subsequent incubation with PKA.
4. Dephosphorylation of tau prior to incubation with kinases significantly altered the influence of individual and multiple kinase incubation on tau antigenicity in a site-specific manner, indicating that prior in situ phosphorylation events markedly influenced subsequent cell-free phosphorylation.
5. In addition to considerations of the potential impact of tau phosphorylation by individual kinases, these findings extend previous studies which indicate that tau antigenicity, and, presumably, its behavior in situ, is influenced by the sequential and convergent influences of multiple kinases.
Similar content being viewed by others
REFERENCES
Arioka, M., Tsukamoto, M., Ishiguro, K., Kato, R., Sato, K., Imahori, K., and Uchida, T. (1993). Tau protein kinase-1 is involved in the regulation of the normal phosphorylation state of tau protein. J. Neurochem. 60:461–468.
Baudier, J., and Cole, R. D. (1987). Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J. Biol. Chem. 262:17577–17583.
Baudier, J., Lee, S.-H., and Cole, R. D. (1987). Separation of the different microtubule-associated tau proteins from bovine brain and their mode 1 phosphorylation by Ca2+/phospholipid-dependent protein kinase C. J. Biol. Chem. 262:17584–17590.
Baumann, K., Mandelkow, E.-M., Biernat, J., Piwnica-Worms, H., and Mandelkow, E. (1993). Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 336:417–424.
Biemat, J., Mandelkow, E.-M., and Schroter, C. (1992). The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule-binding region. EMBO J. 11:1593–1597.
Blanchard, B. J., Raghunandan, R. D., Roder, H. M., and Ingram, V. M. (1994). Hyperphosphorylation of human tau by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: Relation to Alzheimer's disease. Biochem. Biophys. Res. Commun. 200:187–194.
Brambett, G. T., Goedert, M., Jakes, R., Merrick, S. E., Trojanowski, J. Q., and Lee, V. M.-Y. (1993). Abnormal tau phosphorylation at ser-396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099.
Boyce, J. J., and Shea, T. B. (1997). Phosphorylation events mediated by protein kinase Cα and ε participate in regulation of tau steady-state levels and generation of certain “Alzheimer-like” phospho-epitopes. Int. J. Dev. Neurosci. 15:295–307.
Cressman, C. M., and Shea, T. B. (1995). Hyperphosphorylation of tau and filopodial retraction following microinjection of protein kinase C catalytic subunits. J. Neurosci. Res. 42:648–656.
Cressman, C. M., Mercken, M. M., and Shea, T. B. (1995). Alteration in tau antigenicity and electrophoretic migration by PKC under cell-free conditions. Neurosci. Res. Commun. 17:61–64.
Goedert, M., Jakes, R., Crowther, R. A., Six, J., Lubke, U., Vandermeeren, M., Cras, P., Trojanowski, J. Q., and Lee, V. M.-Y. (1993a). The abnormal phosphorylation of tau proteins at ser-202 in Alzheimer's disease recapitulates phosphorylation during development. Proc. Natl. Acad. Sci. USA. 90:5066–5070.
Goedert, M., Jakes, R., Crowther, R. A., Six, J., Lubke, U., Vandermeeren, M., Cras, P., Trojanowski, J. Q., and Lee, V. M.-Y. (1993b). The abnormal phosphorylation of tau proteins at ser-202 in Alzheimer's disease recapitulates phosphorylation during development. Proc. Natl. Acad. Sci. USA 90:5066–5070.
Goedert, M., Jakes, R., Crowther, R. A., Cohen, P., Vanmechelen, E., Vandermeeren, M., and Cras, P. (1994a). Epitope mapiated protein tau are dephosphorylated by protein phosphatase 2A-1. FEBS Lett. 312:95–99.
Goedert, M., Jakes, R., Crowther, R. A., Cohen, P., Vanmechelen, E., Vandermeeren, M., and Cras, P. (1994b). Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: Identification of phosphorylation sites in tau protein. Biochem. J. 301:871–877.
Hanger, D. P., Hughes, K., Woodgett, J. R., Brion, J. P., and Anderton, B. H. (1992). Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localization of the kinase. Neurosci. Lett. 147:58–62.
Hollingsworth, E. B., Ukena, D., and Daly, J. W. (1986). The protein kinase C activator phorbol-12myristate-13-acetate enhances cyclic AMP accumulation in pheochromocytoma cells. FEBS Lett. 196:131–134.
Kobayashi, S., Ishiguro, K., Omori, A., Takamatsu, M., Arioka, M., Imahora, K., and Uchida, T. (1993). A cdc-related kinase PSSALRE/cdk5 is homologous with the 30kDa subunit of tau protein kinase-1, a proline-directed kinase associated with microtubules. FEBS Lett. 335:171–175.
Kosik, K. S., and Greenberg, S. M. (1994). Tau protein and Alzheimer's disease. In Terry, R. D., Katzman, R., and Bick, K. L. (eds.), Alzheimer's Disease, Raven Press, New York, pp. 335–344.
Lang, E., and Otvos, L. (1992). A serine-proline change in the Alzheimer's disease-associated epitope Tau-2 results in altered secondary structure, but phosphorylation overcomes the conformational gap. Biochem. Biophys. Res. Commun. 188:162–169.
Latimer, D. A., Gallo, J.-M., Lovestone, S., Miller, C. C. J., Reynolds, C. H., Marquardt, B., Stable, S., Woodgett, J. R., and Anderton, B. H., (1995). Stimulation of MAP kinase by v-raf transformation of fibroblasts fails to induce hyperphosphorylation of transfected tau. FEBS LETT. 365:42–46.
Ledesma, M. D., Correas, L., Avila, J., and Diaz-Nido, J. (1992). Implication of brain cdc2 and MPA kinases in the phosphorylation of tau protein in Alzheimer's disease. FEBS Lett. 308:218–224.
Liu, W.-K., Moore, W. T., Williams, R. T., Hall, F. L., and Yen, S.-H. (1993). Application of synthetic phospho-and unphospho-peptides to identify phosphorylation sites in a subregion of the tau molecules, which is modified in Alzheimer's disease. J. Neurosci. Res. 34:371–376.
Lu, W., Soria, J. P., and Wood, J. G. (1993). p44mpk MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J. Neurosci. Res. 35:439–444.
Mandelkow, E.-M., Drewes, G., Biernat, J., Gustke, N., Van Lint, J., Vandenheede, J. R., and Mandelkow, E. (1992). Glycogen synthase kinase 3 and the Alzheimer's disease-like state of microtubule-associated protein tau. FEBS Lett. 314:315–321.
Mandelkow, E.-M., Biernat, J., Drewes, G., Gustke, N., Trinczek, B., and Mandelkow, E. (1995). Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 16:355–362.
Morishima-Kawashima, M., Hasegawa, M., Takio, K., Suzuki, M., Yoshida, H., Titani, K., and Ihara, Y. (1995). Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270:823–829.
Mulot, S. F. C., Hughes, K., Woodgett, J. R., Anderton, B. H., and Hanger, D. P. (1994). PHF-tau from Alzheimer's brain comprises four species on SDS-PAGE which can be mimicked by in vitro phosphorylation of human brain tau by glycogen synthase kinase-3β. FEBS Lett. 349:359–364.
Paudel, H. K., Lew, J., Ali, Z., and Wang, J. H. (1993). Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J. Biol. Chem. 268:23512–23518.
Pelech, S. L. (1995). Networking with proline-directed protein kinases implicated in tau phosphorylation. Neurobiol. Aging 16:247–261.
Pelech, S. L., and Sangria, J. S. (1992). Mitogen-activated protein kinases: Versatile transducers for cell signaling. Trends Biochem. Soc. 17:233–238.
Peraldi, P., Frodin, M., Barnier, J. V., Calleja, V., Scimeca, J.-C., Filloux, C., Calothy, G., and Van Obberghen, E. (1995). Regulation of the MAP kinase cascade in PC12 cells: B-Raf activates MEKI (MAP kinase or ERK kinase) and is inhibited by cAMP. FEBS Lett. 357:290–296.
Pundreddy, S., and Shea, T. B. (1997). AD-like tau phosphorylation in human neuroblastoma cells following PKC hyperactivation is mediated by MAP kinase. Neurosci. Res. Commun. 21:173–177.
Raghunandan, R., and Ingram, V. M. (1995). Hyperphosphorylation of the cytoskeletal protein tau by the MAP-kinase PK40erk: Regulation by prior phosphorylation with cAMP-dependent protein kinase A. Biochem. Biophys. Res. Commun. 215:1056–1066.
Sengupta, A., Wu, Q., Grundkle-Iqbal, I., Iqbal, K., and Singh, T. J. (1997). Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol. Cell. Biochem. 167:99–105.
Shea, T. B. (1997). Phosphatidyl serine alters tau antigenicity, phosphorylation, proteolysis and association with microtubules: Implication for tau function under normal and degenerative conditions. J. Neurosci. Res. 50:114–122.
Shea, T. B., and Beermann, M. L. (1994). Respective roles of neurofilaments, microtubules, MAP1B and tau in the outgrowth and stabilization of axonal neurites. Mol. Biol. Cell. 5:863–875.
Shea, T. B., Beermann, M. L., Nixon, R. A., and Fischer, I. (1992a). Microtubule-associated protein tau is required for axonal neurite elaboration by neuroblastoma cells. J. Neurosci. Res. 32:363–374.
Shea, T. B., Beermann, M. L., Leli, U., and Nixon, R. A. (1992b). Opposing influences of protein kinase activities on neurite outgrowth in human neuroblastoma cells: Initiation by kinase A and restriction by kinase C. J. Neurosci. Res. 33:398–407.
Shea, T. B., Spencer, M. J., Beerman, M. L., Cressman, C. M., and Nixon, R. A. (1996). Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: Involvement of calpain-mediated hydrolysis of protein kinase. C. J. Neurochem. 66:1539–1549.
Singh, T. J., Zaidi, T., Grundke-Iqbal, I., and Iqbal, K. (1994). Modulation of GSK-3-catalyzed phosphorylation of microtuble-associated protein tau by non-proline-dependent protein kinases. FEBS Lett. 358:4–8.
Singh, T. J., Haque, N., Grundke-Iqbal, I., and Iqbal, K. (1994b). Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline dependent kinases and GSK-3. FEBS Lett. 358:267–272.
Steiner, B., Mandelkow, E.-M., Biernat, J., Gustke, N., Meyer, H. E., Schmidt, B., Mieskes, G., Dreschsel, D., Kirschner, M. W., Goedert, M., and Mandelkow, E. (1990). Phosphorylation of microtubule-associated protein tau: Identification of the site for Ca2+-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 9:3539–3544.
Szendrei, G. I., Lee, V. M.-Y., and Otvos, L. (1993). Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J. Neurosci. Res. 34:243–249.
Trojanowski, J. Q., Schmidt, M. L., Shin, R.-W., Bramblett, G. T., Rao, D., and Lee, V. M.-Y. (1993a). Altered tau and neurofilament proteins in neurodegenerative diseases: Diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 3:45–54.
Trojanowski, J. Q., Schmidt, M. L., Shin, R.-W., Bramblett, G. T., Goedert, M., and Lee, V. M.-Y. (1993b). PHF tau (A68): From pathological marker to potential mediator of neuronal dysfunction and degeneration in Alzheimer's disease. Clin. Neurosci. 1:184–191.
Vulliet, R., Halloran, S. M., Braun, R. K., Smith, A. J., and Lee, G. (1992). Proline-directed phosphorylation of human tau protein. J. Biol. Chem. 267:22570–22574.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shea, T.B., Cressman, C.M. The Order of Exposure of Tau to Signal Transduction Kinases Alters the Generation of “AD-Like” Phosphoepitopes. Cell Mol Neurobiol 19, 223–233 (1999). https://doi.org/10.1023/A:1006977127422
Issue Date:
DOI: https://doi.org/10.1023/A:1006977127422