Abstract
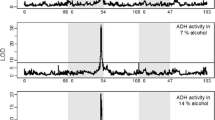
Thirty-five cryptic variant lines were used to examine the mechanisms involved in genetic modulation of alcohol metabolism in Drosophila. Late third-instar larval, preemergence pupal, and adult stages cultured at 18 and 28 C were examined. Spectrophotometric analyses for native alcohol dehydrogenase (ADH) activity and residual ADH activity after treatment with guanidine hydrochloride and heat were performed. Differential response of cryptic variants to treatment with the denaturants during development suggested that this variation may have an adaptive significance.
Similar content being viewed by others
References
Avise, J. C., and McDonald, J. F. (1976). Enzyme changes during development of holo- and hemimetabolic insects. Comp. Biochem. Physiol. 53B393.
Birley, A. J., and Barnes, B. W. (1973). Genetical variation for enzyme activity in a population of Drosophila melanogaster. I. Extent of the variation for alcohol dehydrogenase activity. Heredity 31413.
Courtright, J. B. (1976). Drosophila gene-enzyme systems. Adv. Genet. 18249.
Dickinson, W. J., and Sullivan, D. T. (1975). The study of gene-enzyme systems in Drosophila. In Results and Problems in Cell Differentiation. Vol. 6 Springer-Verlag, New York.
Gionfriddo, M. A., and Vigue, C. L. (1978). Drosophila alcohol dehydrogenase frequencies and temperature. Genet. Res. 3197.
Hewitt, N. E., Pipkin, S. B., Williams, N., and Chakrabarty, P. K. (1974). Variation in ADH activity levels in class I and class II strains of Drosophila. J. Hered. 65141.
Johnson, F. M., and Schaffer, H. E. (1973). Isozyme variability in species of the genus Drosophila. VII. Genotype-environment relationships in populations of Drosophila melanogaster from eastern United States. Biochem. Genet. 10149.
Johnson, G. B. (1974). Enzyme polymorphism and metabolism. Science 18428.
Koehn, P. K., and Eanes, W. F. (1979). Molecular structure, polypeptide size and genetic variation of enzymes. Isozymes Curr. Topics Biol. Med. Res. 3185.
Kojima, K. I., and Tobari, Y. N. (1969). The pattern of viability changes associated with genotype frequency at the alcohol dehydrogenase locus in a population of Drosophila melanogaster. Genetics 61201.
Kojima, K. I., Gillespie, J., and Tobari, Y. N. (1970). A profile of Drosophila species' enzymes assayed by electrophoresis. I. Number of alleles, heterozygosities, and linkage disequilibrium in glucose-metabolizing systems and some other enzymes. Biochem. Genet. 4627.
McDonald, J. F., and Avise, J. C. (1976). Evidence for the adaptive significance of enzyme activity levels: Interspecific variation in α-GPDH and ADH in Drosophila. Biochem. Genet. 14347.
McDonald, J. F., and Ayala, F. J. (1978). Gene regulation in adaptive evolution. Can. J. Genet. Cytol. 20159.
Miglani, G. S. (1980). Regulatory Properties of Cryptic Variants at the Alcohol Dehydrogenase (Adh) Locus in Drosophila melanogaster, Ph.D. dissertation, Howard University, Washington, D.C., p. 145.
O'Brien, S. L., and McIntyre, R. J. (1972a). The α-glycerophosphate cycle in Drosophila melanogaster. I. Biochemical and developmental aspects. Biochem. Genet. 7141.
O'Brien, S. J., and McIntyre, R. J. (1972b). The α-glycerophosphate cycle in Drosophila melanogaster. II. Genetic aspects. Genetics 71127.
Pipkin, S. B., and Hewitt, N. E. (1972). Variation of alcohol dehydrogenase levels in Drosophila species hybrids. J. Hered. 63267.
Pipkin, S. B., Rhodes, C., and Williams, N. (1973). Influence of temperature on alcohol dehydrogenase polymorphism. J. Hered. 64181.
Pipkin, S. B., Potter, J. H., Lubega, S., and Springer, E. (1975). Further studies on alcohol dehydrogenase polymorphism in Mexican strains of Drosophila melanogaster. In Markert, C. L. (ed.), Isozymes. IV. Genetics and Evolution Academic Press, New York, p. 547.
Pipkin, S. B., Springer, E., Law, S., and Lubega, S. (1976). New studies of the alcohol dehydrogenase cline in D. melanogaster from Mexico. J. Hered. 67258.
Thompson, J. N., Jr., and Kaiser, T. N. (1977). Selection upon slow-migrating Adh alleles differing in enzyme activity. Heredity 38191.
Ursprung, H., and Carlin, L. (1968). Drosophila alcohol dehydrogenase: In vitro changes of isozyme patterns. Ann. N.Y. Acad. Sci. 151456.
Ward, R. D. (1974). Alcohol dehydrogenase in Drosophila melanogaster: Activity variation in natural populations. Biochem. Genet. 12449.
Ward, R. D., and Herbert, P. D. N. (1972). Variability of alcohol dehydrogenase activity in a natural population of Drosophila melanogaster. Nature New Biol. 236243.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miglani, G.S., Ampy, F.R. Drosophila alcohol dehydrogenase: Developmental studies on cryptic variant lines. Biochem Genet 19, 947–954 (1981). https://doi.org/10.1007/BF00504259
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00504259