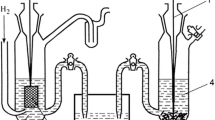
Summary
We present here a calorimetric method and the construction details of a differential calorimeter useful for studying the reactions in an electrolytic cell and more generally slow chemico-physical processes occurring in thermodynamically open systems. The method allows measurements of the heat balance of the cell, from which the enthalpy change of the process under investigation can be calculated. The theoretical description of the calorimetric cell and the results of several studies planned to describe the performances of the instrument up to the boiling point of the electrolytic solution are reported. The features of this calorimeter fulfil most of the requirements of ≪col fusion≫ experiments, where the heat production is the fundamental and controversial aspect. By controlling both the heat and the matter exchanged, the calorimeter can be utilised also to study bioenergetic processes,e.g. fermentation, microbial metabolism and biodegradation, and liquid phase chemical reactions, involving gases as reactants and/or products.
Similar content being viewed by others
References
Fleischmann M. andPons S.,J. Electroanal. Chem.,261 (1989) 301.
Fleischmann M. andPons S.,Phys. Lett. A,176 (1993) 118.
Srinivasan M.,Curr. Sci.,60 (1991) 447;Storms E.,Fusion Technol.,20 (1991) 433.
McKubre M. C. H., Crouch-Baker S., Rocha-Filho R. C., Smedley S. I., Tanzella F. L., Passell T. O. andSantucci J.,J. Electroanal. Chem.,368 (1994) 55.
Miles M. H., Bush B. F. andStilwell D. E.,J. Phys. Chem.,98 (1994) 1948.
Shkedi Zvi, McDonald Robert C., Breen John J., Maguire Stephen J. andVeranth Joe,Fusion Technol.,8 (1995) 1720.
Barbini A., Bertolini D., Cassettari M., Papucci F., Salvetti A., Salvetti G. andVeronesi S.,Rev. Sci. Instrum.,60 (1989) 1308.
Bertolini D., Cassettari M., Salvetti G., Tombari E. andVeronesi S.,Rev. Sci. Instrum.,61 (1990) 2416.
Cassettari M., Papucci F., Salvetti G., Tombari E., Veronesi S. andJohari G. P.,Rev. Sci. Instrum.,64 (1993) 1076.
Weast R. C. (Editor),Handbook of Physics and Chemistry, 60th edition (CRC Press Inc.) 1980.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrari, C., Papucci, F., Salvetti, G. et al. A calorimeter for the electrolytic cell and other open systems. Il Nuovo Cimento D 18, 1333–1346 (1996). https://doi.org/10.1007/BF02453267
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02453267