Abstract
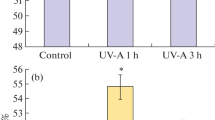
Dry seeds of Leucadendron laureolum (Lam.) Fourc. (Proteaceae) were exposed for different intervals (range: 7 to 84 days) to visible, UV-A and UV-B radiation of different biologically effective dose (range: 0 to 11.43 kJ m-2 d-1). Changes in seed germination, physiology and ultrastructure, and residual UV effects on seedling performance, were examined. Germination was depressed in seeds following short (7-day) exposures to UV radiation. This depression was intensified with increased UV exposure dose, and most pronounced at shorter UV-B wavelengths. Also glutathione reductase (GR) activities increased in seeds exposed to shorter UV-B wavelengths, but these were unaffected by irradiation dose level in the UV-B range. Electrolyte leakage rates from UV-irradiated seeds were unaltered, which indicated that germination depression did not result from intrinsic membrane damage. The reversal of germination depression (UV-induced dormancy) in UV-irradiated seeds by red light pointed to the possible involvement of phytochrome in this photo-response. Germination depression disappeared in seeds after 56-days irradiation, possibly due to photoreceptor damage by excess UV light. At this stage, all UV irradiated seeds, irrespective of treatment wavelength or dose level, exhibited increased electrolyte leakage rates, which indicated membrane perturbation. Also, increased GR activities were observed in irradiated seeds, but these were proportionately smaller in seeds exposed to shorter wavelength UV-B radiation (9.1 to 35.8% increase) than longer wavelength UV-A (73.4% increase) and visible (97.7% increase) radiation. This implied a metabolic limitation for scavenging of free radicals and peroxides in aging seeds exposed to UV-B radiation, which pointed to accelerated seed deterioration. It was indirectly supported by ultrastructural evidence of sub-cellular damage (lipid coagulation and plasmalemma withdrawal from cell walls) in embryonic tissues of seeds after 84 days UV-B exposure, and reflected in decreased leaf numbers, photochemical efficiencies, and foliar chlorophyll a and carotenoid levels in seedlings cultured from these seeds.
Similar content being viewed by others
References
Alenius, C. M., Vogelmann, T. C. & Bornman, J. F. 1995. A threedimensional representation of the relationship between penetration of u.v.-B radiation and u.v.-screening pigments in leaves of Brassica napus. New Phytol. 131: 297-302.
Ballare, C. L., Barnes, P. W. & Flint, S. D. 1995. Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolated tomato seedlings. I. The photoreceptor. Physiol. Plant. 93: 584- 592.
Bewley, J. D. & Black, M. 1982. Physiology and Biochemistry of Seeds in relation to germination, Vol. 2. Springer-Verlag, Berlin.
Blumthaler, M., Ambach, W., Silbernagl, R. & Staehelin, J. 1994. Erythemal UV-B irradiance (Robertson-Berger sunburn meter data) under ozone deficiencies in winter/spring 1993. Photochem. Photobiol. 59: 657-659.
Bond, W. J. 1984. Fire survival in Cape Proteaceae-influence of fire season and seed predators. Vegetatio 56: 65-74.
Bond, W. J. 1985. Canopy-stored seed reserves (serotiny) in Cape Proteaceae. South African J. Bot. 51: 181-186.
Bowler, C., van Montagu, M. & Inze, D. 1992. Superoxidase dismutase and stress tolerance. Ann. Rev. Plant Physiol. Plant Molec. Biol. 43: 83-116.
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254.
Brits G. J. 1987. Germination depth vs.temperature requirements in naturally dispersed seeds of Leucospermum cordifoliumand L. cuneiforme(Proteaceae). South African J. Bot. 53: 119-124.
Bucharov, P. & Gantcheff, Ts. 1984. Influence of accelerated and natural aging on free radicle levels in soybean seeds. Physiol. Plant. 60: 53-56.
Caldwell, M. M. 1971. Solar ultraviolet radiation and the growth and development of higher plants. Pp. 131-177. In: Giese, A.C. (ed.), Photophysiology, Vol. 6. Academic Press, New York.
Czabator, F. J. 1962. Germination value: an index combining speed and completeness of pine seed germination. Forest Sci. 8: 386- 395.
Demmig-Adams, B. & Adams III, W.W. 1992. Photoprotection and other responses of plants to high light stress. Ann. Rev. Plant Physiol. Plant Molec. Biol. 43: 599-626.
Drennan, P. M. & Berjak, P. 1982. Degeneration of the salt glands accompanying foliar maturation in Avicennia marina(Forsk.) Vierh. New Phytol. 90: 165-176.
Foyer, C. H. & Halliwell, B. 1976. The presence of glutathione and GR in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21-25.
Foyer, C. H., Lelandais, M. & Kunert, K. J. 1994. Photooxidative stress in plants. Physiol. Plant. 92: 696-717.
Glyphis, J. P. & Puttick, G. M. 1988. Phenolics in some South African mediterranean shrubland plants. Phytochemistry 27: 743 -751.
Green, A. E. S. 1983. The penetration of ultraviolet radiation to the ground. Physiol. Plant. 58: 351-359.
Hendry, G. A. F. 1993. Oxygen, free radicle processes and seed longevity. Seed Sci. Res. 3: 141-153.
Herman, J. R. & Larko, D. 1994. Low ozone amounts during 1992-1993 from Nimbus 7 and Meteor 3 total ozone mapping spectrometers. J. Geophys. Res. 98: 12783-12793.
Hon, D. N. S. 1991. Photochemistry of wood. Pp. 525-555. In: Hon, D. N. S. & Shiraishi, N. (eds), Wood and Cellulosic Chemistry. Marcel Dekker, Inc., New York.
Hon, D. N. S. 1994. Degradative effects of ultraviolet light and acid rain on wood surface quality. Wood Fibre Sci. 26: 185-191.
Hon, D. N. S. & Feist, W. C. 1993. Interaction of sulphur dioxide and nitric oxide with photoirradiated wood surfaces. Wood Fibre Sci. 25: 136-141.
Jablonski, P. P. & Anderson, J. W. 1984. Role of flavonoid in the peroxide-dependent oxidation of glutathione catalyzed by pea extract. Phytochemistry 23: 1865-1869.
Kerr, J. B. & McElroy, C. T. 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262: 1032-1034.
Kitajima, M. & Butler, W. L. 1975. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochem. Biophys. Acta 376: 105-115.
Kruger, F. J. & Bigalke, R. C. 1984. Fire in fynbos. Pp. 67-114. In: de Booysen, P. & Tainton, N. M. (eds), Ecological Effects of Fire in South African Ecosystems, Ecological Studies, Vol. 48. Springer-Verlag, Berlin.
Le Maitre, D. C. 1988. The effect of parent density and season of burn on the regeneration of Leucadendron laureolum(Proteaceae) in the Kogelberg. South African J. Bot. 54: 581- 584.
Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth. Enzymol. 148: 350- 382.
Lichtenthaler, H. K. & Rinderle, U. 1988. The role of chlorophyll fluorescence in the detection of stress conditions in plants. SCRC Critical Rev. Analyt. Chem. 19(Suppl. 1): 529-585.
Lowe, N. J. & Shaath, N. A. 1990. Sunscreens: Development, evaluation and regulatory aspects. Cosmetic Science and Technology Series 10. Marcel Dekker, New York.
Mirecki, R. M. & Teramura, A. H. 1984. Effects of ultraviolet-B irradiance on soybean. V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 74: 475-480.
Musil, C. F. 1994. Ultraviolet-B irradiation of seeds affects photochemical and reproductive performance of the arid-environment ephemeral Dimorphotheca pluvialis. Env. Exp. Bot. 34: 371- 378.
Mustart P. J. & Cowling R. M. 1991. Seed germination in four serotinous Agulhas Plain Proteaceae. South African J. Bot. 57: 310-313.
Oquist, G. & Wass, R. 1988. A portable, microprocessor operated instrument for measuring chlorophyll fluorescence kinetics in stress physiology. Physiol. Plant. 73: 211-217.
Ponquett, R. T., Smith, M. T. & Ross, G. 1992. Lipid autoxidation and seed ageing: putative relationships between seed longevity and lipid stability. Seed Sci. Res. 2: 51-54.
Pratt, L. H. & Butler, W. L. 1970. Phytochrome conversion by ultraviolet light. Photochem. Photobiol. 11: 503-509.
Priestley, D. A. & Leopold, A. C. 1983. Lipid changes during natural aging of soybean seeds. Physiol. Plant. 59: 467-470.
Puntarulo, S. & Boveris, A. 1990. Effect of natural and accelerated aging on the hydroperoxide metabolism of soybean embryonic axes. Plant Sci. 68: 27-32.
Quaite, F. E., Sutherland, B. M. & Sutherland, J. C. 1992. Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion. Nature 358: 576-578.
Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron opaque stain for electron microscopy. J. Cell Biol. 17: 208- 212.
Rozema, J., van de Staaij, J., Bjorn, L. O. & Caldwell, M. 1997. UVB as an environmental factor in plant life: stress and regulation. Trends Ecol. Evol. 12: 22-29.
Schreiber, U. & Bilger, W. 1993. Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. Prog. Bot. 54: 151-173.
Smith, I. K., Vierheller, T. L. & Thorne, C. A. 1989. Properties and functions of glutathione reductase in plants. Physiol. Plant. 77: 449-456.
Spurr, A. R. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastr. Res. 26: 31-43.
Stewart, R. R. C. & Bewley, J. D. 1980. Lipid peroxidation associated with accelerated aging of soybean axe. Plant Physiol. 65: 245-248.
Sung, J. M. 1996. Lipid peroxidation and peroxide-scavenging in soybean seeds during aging. Physiol. Plant. 9: 85-89.
Teramura, A. H. & Sullivan, J. H. 1994. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 39: 463-473.
Tevini, M. 1993. UV-B Radiation and Ozone Depletion: Effects on Humans, Animals, Plants, Microorganisms, and Materials. Lewis Publishers, Boca Raton, Florida.
Valenzeno, D. P. (1987) Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem. Photobiol. 46: 147-160.
Villiers, T. A. Ultrastructural changes in seed dormancy and senescence. 1980. Pp. 39-65. In: K. V. Thimann (ed.), Senescence in Plants. CRC Series in Aging. Germex, Boca Raton, Florida.
Wang, S. Y., Hong, J. J. & Faust, M. 1991. Changes in ascorbate, glutathione, and related enzyme activities during thidiazuroninduced bud break of apple. Physiol. Plant. 82: 231-236.
Wilson, D. O. & McDonald, M. B. 1986. The lipid peroxidation model of seed ageing. Seed Sci. Technol. 14: 269-300.
van Staden, J. & Brown, N. A. C. 1977. Studies on the germination of South African Proteaceae-a review. Seed Sci. Technol. 5: 633-643.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Musil, C.F., Newton, R.J. & Farrant, J.M. Ultraviolet irradiation effects on serotinous shape Leucadendron laureolum seeds: altered seed physiology and ultrastructure, and seedling performance. Plant Ecology 139, 25–34 (1998). https://doi.org/10.1023/A:1009750404120
Issue Date:
DOI: https://doi.org/10.1023/A:1009750404120