Summary
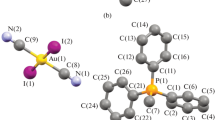
The structure of [Ir(NO)(phen)(PPh3)2][PF6]2 has been determined from x-ray diffractometer data. The compound crystallizes in space groupPnam with four molecules in a unit cell witha = 19.924(12),b = 14.793(9) andc = 16.348(9) A. Full-matrix least-squares refinement has led to a final R value of 0.061 for the 4796 observed reflections. The structure consists of well-separated ions, and the geometry around the metal is trigonal bipyramidal with nitrosyl and bidentate 1,10-phenanthroline (in spite of the very narrow bite angle of 75.8°) ligands occupying the equatorial positions and the triphenylphosphine ligands the axial positions. The cation has an imposed crystallographicm symmetry. Important bond lengths are as follows: Ir-P, 2.391(3): Ir-N (nitrosyl) 1.700(12): Ir-N (1,10-phenanthroline) 2.103(12) and 2.142(11): N-O, 1.201(18)A. The nitrosyl ligand is linear [Ir-N-O = 179.9(9)°] so that this complex can be formulated as an NO+ complex of iridium(I).
Similar content being viewed by others
References
B. A. Frenz and J. A. Ibers,M.T.P. Int. Rev. Sci. Phys. Chem. Ser. One, 11, 33 (1972).
J. P. Collman, P. Farnham and G. Dolcetti,J. Am. Chem. Soc., 93, 1788 (1971).
J. A. McGinnety,M.T.P. Int. Rev. Sci. Phys. Chem. Ser. One, 5, 229 (1972).
B. L. Haymore and J. A. Ibers,Inorg. Chem., 14, 2610 (1975).
C. G. Pierpont and R. Eisenberg,Inorg. Chem., 12, 199 (1973).
J. H. Enemark, R. D. Feltham, J. Riker-Nappier and K. F. Bizot.Inorg. Chem., 14, 624 (1975).
J. H. Enemark, R. D. Feltham, B. T. Huie, P. L. Johnson and K. Bizot Swedo,J. Am. Chem. Soc., 99, 3285 (1977).
M. Ghedini, G. Dolcetti, O. Gandolfi and B. Giovannitti,Inorg. Chem., 15, 2385 (1976).
M. Ghedini, G. Dolcetti and G. Denti,Transition Met. Chem., 3, 177 (1978).
R. Hoffmann, M. M. L. Chen, M. Elian, A. R. Rossi and D. M. P. Mingos,Inorg. Chem., 13, 2666 (1974).
F. Basolo and R. G. Pearson,Mechanism of Inorganic Reactions, 2nd Edit., Wiley, New York, 1967, p. 374.
B. A. Frenz and J. A. Ibers,Inorg. Chem., 11, 1109 (1972) and refs. therein.
M. Lanfranchi, A. Tiripicchio. M. Ghedini and G. Dolcetti,Transition Met. Chem., in press.
L. Johansson, M. Molund and A. Oskarsson,Inorg. Chim. Acta, 31, 117 (1978) and refs. therein.
L. Pauling,The Nature of the Chemical Bond, Cornell University Press, Ithaca, N. Y., 1967, p.127.
G. Sheldrich,System of Computing Programs, University of Cambridge (1976).
International Tables for X-Ray Crystallography, Vol. IV, Kynoch Press, Birmingham, 1974.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tiripicchio, A., Tiripicchio Camellini, M., Ghedini, M. et al. Crystal and molecular structure of nitrosyl(1,10-phenanthroline)-bis(triphenylphosphine)iridium(I) Dihexafluorophosphate, [Ir(NO)(phen)(PPh3)2][PF6]2 . Transition Met Chem 5, 102–105 (1980). https://doi.org/10.1007/BF01396881
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01396881