Abstract
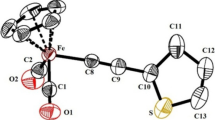
A series of substituted tricarbonyl(trimethylenemethane)-iron complexes were prepared by functionalization of (3-butenyltrimethylenemethane)Fe(CO)3 (3) or (formyltrimethylenemethane)Fe(CO)3 (14). The products are characterized by 1H and 13C-n.m.r., i.r. and high resolution mass spectroscopy. In addition, the X-ray diffraction analysis of one of these derivatives (13a) was accomplished. Reactions of (3), which introduce a new chiral centre, occur in a non-diastereoselective fashion, while reactions of (14) that introduce a new chiral centre proceed with good diastereoselectivity. The remote nature of the reactive functionality and the (TMM)Fe(CO)3 group is responsible for the lack of diastereoselectivity for (3). The present work demonstrates the robust nature of the (TMM)Fe(CO)3 fragment, embodied in its resistance toward oxidation, and to attack by nucleophiles.
Similar content being viewed by others
References
P. Dowd, A. Gold and K. Sachdev, J. Am. Chem. Soc., 90, 2715 (1968) and refs cited therein.
H. C. Louguet-Higgins and L. E. Orgel, J. Chem. Soc., 1969 (1956).
(a) G. F. Emerson, K. Ehrlich, W. P. Giering and P. C. Lauterbur, J. Am. Chem. Soc., 88, 3172 (1966); (b) K. Ehrlich and G. F. Emerson, J. Am. Chem. Soc., 94, 2464 (1972); (c) J. S. Ward and R. Pettit, J. Chem. Soc., Chem. Commun., 1419 (1970); (d) S. G. Allen, S. G. Barnes, M. Green, G. Moran, L. Trollope, N. W. Murrall, A. J. Welch and D. M. Sharaiha, J. Chem. Soc., Dalton Trans., 1157 (1984); (e) G.-M. Su, G.-H. Lee, S. M. Peng and R.-S. Liu, J. Chem. Soc., Chem. Commun., 215 (1992); (f) M. D. Jones and R. D. W. Kemmitt, J. Chem. Soc., Chem. Commun., 811 (1985); (g) M. D. Jones, R. D. W. Kemmitt and A. W. G. Platt, J. Chem. Soc., Dalton Trans., 1411 (1986); (h) B. M. Trost, Angew. Chem., Int. Ed. Engl., 25, 3 (1986) and refs cited therein (i) C.-C. Su, J.-T. Chen, G.-H. Lee and Y. Wang, J. Am. Chem. Soc., 116, 4999 (1994); (j) P. W. Blosser, D. G. Schimpff, J. C. Gallucci and A. Wojcicki, Organometallics, 12, 1993 (1993).
J. A. Mondo and J. A. Berson, J. Am. Chem. Soc., 105, 3340 (1983).
M. Franck-Neumann and A. Kastler, Synlett, 61 (1995).
M. Franck-Neumann, D. Martina and M. P. Heitz, Tetrahedron Lett., 30, 6679 (1989).
(a) W. A. Donaldson, M. A. Hossain and C. D. Cushnie, J. Org. Chem., 60, 1611 (1995); (b) W. A. Donaldson and M. A. Hossain Tetrahedron Lett., 33, 4107 (1992).
M. Franck-Neumann, A. Kastler and P.-J. Colson, Tetrahedron Lett., 33, 7051 (1991).
E. S. Magyar and C. P. Lillya, J. Organometal. Chem., 116, 99 (1976).
A. Almenningen, A. Haaland and K. Wahl, Acta Chem. Scand., 23, 1145 (1969).
M. R. Churchill and K. Gold, Inorg. Chem., 8, 401 (1969).
L. Girard, J. H. MacNiel, A. Mansour, A. C. Chiverton, J. A. Page, S. Fortier and M. C. Baird, Organometallics, 10, 3114 (1991).
V. G. Young, Jr, P. Kleindl and W. A. Donaldson, Bull. Soc. Chim. Belg., 106, 175 (1997).
M. Franck-Neumann, P. Stöber and G. Passmore, Tetrahedron: Asymmetry, 7, 3193 (1996).
B. R. Bonazza, C. P. Lillya, E. S. Magyar and G. Scholes, J. Am. Chem. Soc., 101, 4100 (1979).
P. J. Kleindl and W. A. Donaldson, J. Org. Chem., 62, 4176 (1997).
(TMM)Fe(CO)3 complexes have been shown to be stable to MnO2 oxidation(5,8) and to ozonolysis conditions(5).
J. B. Weinrach and D. W. Bennett, J. Appl. Crystallogr., 24, 91 (1991).
E. J. Gabe, Y. Le Page, J.-P. Charland, R. L. Lee and P. S. White, J. Appl. Crystallogr., 22, 384 (1989).
G. M. Sheldrick, Acta Crystallogr., A46, 467 (1990).
G. M. Sheldrick, A Program for Crystal Structure Solution, Institute für Anorganishe Chemie der Universtität, Göttingen, FRG.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Donaldson, W.A., Cushnie, C.D., Guo, S. et al. Synthesis and characterization of tricarbonyl(trimethylenemethane)iron complexes: crystal structure of (2-methylene-6-p-nitrobenzoyloxy-heptan-1,3-diyl)Fe(CO)3. Transition Metal Chemistry 22, 592–596 (1997). https://doi.org/10.1023/A:1018521009872
Issue Date:
DOI: https://doi.org/10.1023/A:1018521009872