Abstract
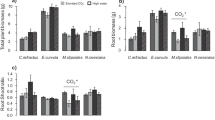
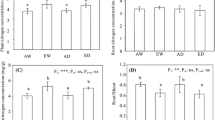
Root growth and physiological uptake capacity for NH +4 and NO −3 were examined for seedlings of loblolly and ponderosa pine grown for 160 days under two CO2 levels, ambient (35 Pa) and ambient plus 35 Pa (70 Pa). Fraction of biomass allocated to active fine roots as well as total N (NH +4 + NO −3 ) absorption per unit root dry mass were unaffected by CO2. On a whole-plant basis, elevated CO2 led to a significant increase in N acquisition in loblolly but not in ponderosa pine. However, even in loblolly pine where CO2 significantly increased plant N acquisition, the relative increase, in biomass far exceeded the gain in N, i.e. a 60% increase in total dry weight was accompanied by only a 30% increase in N gain in response to high CO2. We suggest that the commonly reported decline in tissue N concentration of these and other species at high CO2 is largely caused by inability of the root systems to sufficiently compensate for increased N demand. Elevated CO2 significantly altered root uptake capacity of the different N forms, i.e., high CO2 significantly increased NO −3 absorption rates, but decreased NH +4 absorption rates in both species though the decrease in loblolly was insignificant. However, elevated CO2 increased root respiration rate in loblolly pine while significantly decreasing it in ponderosa pine. This indicates that CO2-induced changes in plant preference for inorganic N forms is not simply regulated by root energy status. If changes in plant preference for inorganic N forms represent typical responses to elevated CO2, the results could have important implications for N dynamics in managed and natural plant communities.
Similar content being viewed by others
References
Aber J D, Nadelhoffer KJ, Steudler P and Mellilo J M 1989 Nitrogen saturation in northern forest ecosystems. Biosci. 39, 378-386.
Allen H, Doughty P M and Campbell R G 1990 Manipulation of water and nutrients practices and opportunity in southern US pine forests. For. Ecol. Manage. 30, 437-453.
Aslam M, Travis R L and Huffaker R C 1994 Stimulation of nitrate and nitrite efflux by ammonium in barley (Hordeum vulgareL.) seedlings. Plant Physiol. 106, 1293-1301.
Baker J B and Langdon O G 1990 Pinus taedaL. InSilvics of North America. Volume 1, Conifers. Eds. R M Burns and B H Honkala. pp 497-512. Agriculture Handbook USDA, Forest Service, Washington, USA.
Barber S A 1984 Soil Nutrient Bioavailability. A Mechanistic Approach. John Wiley and Sons, New York. 398 p.
BassiriRad H, Thomas R B, Reynolds J F and Strain R B 1996 Differential responses of root uptake kinetics of NH +4 and NO -3 to enriched atmospheric CO2 in field-grown loblolly pine. Plant Cell Environ. 19, 367-371.
Baxter R, Gantley M, Ashenden T W and Farrar J F 1994 Effects of elevated carbon dioxide on three grass species from montane pasture. II. Nutrient uptake, allocation and efficiency of use. J. Exp. Bot. 278, 1267-1278.
Binkley D and Hart S C 1989 The components of nitrogen availability assessments in forest soils. Adv. Soil Sci. 10, 57-103.
Blacquiè re T 1987 Ammonium and nitrate nutrition in Plantago lanceolataand P. majorII. Efficiency of root respiration and growth.Comparison ofmeasured and theoretical values of growth respiration. Plant Physiol. Biochem. 25, 1775-1785.
Bloom A J and Finazzo J 1986 The influence of ammonium and chloride on potassium and nitrate absorption by barley roots depends on time of exposure and cultivars. Plant Physiol. 81, 67-69.
Bloom A J, Sukrapanna S S and Warner R L 1992 Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol. 99, 1294-1301.
Boyer W D, Romancier RM and Ralston CW 1971 Root respiration rates of four tree species grown in the field. For. Sci. 17, 492-493.
Chapin F S III 1980 The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 11, 233-260.
Cole D W 1981 Nitrogen uptake and translocation by forest ecosystems. InTerrestrial Nitrogen Cycles. Processes, Ecosystem Strategies and Management Impacts. Eds. F E Clark and T Rosswall. Ecological Bulletin. pp 219-232. Swedish Natural Science Research Council, Stockholm.
Coleman J S, McConnaughay K D M and Ackerly D D 1994 Interpreting phenotypic variation in plants. Tree 9, 187-191.
Coleman J S, McConnaughay KDM and Bazzaz FA, 1993 Elevated CO2 and plant nitrogen-use: is reduced tissue nitrogen concentration size-dependent? Oecologia 93, 195-200.
Christensen N L and MacAller T 1985 Soil mineral nitrogen transformations during succession in the piedmont of North Carolina. Soil Biol. Biochem 17, 675-681.
Conroy J P 1992 Influence of elevated atmospheric CO2 concentrations on plant nutrition. Aust. J. Bot. 40, 445-56.
Curtis, P S, Zak D R, Pregitzer K S and Teeri J A 1994 Above and belowground response of Populus grandidentatato elevated atmospheric CO2 and soil N availability. Plant Soil 165, 45-51
Downs R J and Hellmers H 1975 Controlled climate and plant research. World Meteorological Organization, Tech Note 148, Geneva, Switzerland.
Eamus D and Jarvis P G 1989 The direct effects of increase in the global atmospheric CO2-concentration on natural and commercial trees and forests. Adv. Ecol. Res. 19, 1-55.
Gifford R M, Lambers H and Morison J I L 1985 Respiration of crop species under CO2 enrichment. Physiol. Plant. 63, 351-356.
Glass A D M, Thompson R G and Bordeleau L 1985 Regulation of NO3-influx in barley. Studies using 13NO3-. Plant Physiol. 77, 379–381.
Griffin K L, Winner WE and Strain B R 1995 Growth and dry matter partitioning in loblolly and ponderosa pine seedlings in response to carbon and nitrogen availability. New Phytol. 129, 547-556.
Hanson G C, Groffman P M and Gold A J 1994 Symptoms of nitrogen saturation in a wild riparian wetland. Ecol. Appl. 4, 750-756.
Haynes R J and Goh K M 1978 Ammonium and nitrate nutrition of plants. Biol. Rev. 53, 465-510.
Hellmers H and Giles L J 1979 Carbon dioxide: critique I. InControlled environment guidelines for plant research. Eds. T W Tibbitts and T T Kozlowski. pp 229-234. Academic Press, New York.
Hilbert D W, Larigauderie A and Reynolds J F 1991 The influence of carbon dioxide and daily photon-flux density on optimal leaf nitrogen concentration and root:shoot ratio. Ann. Bot. 68, 365- 376.
Hocking P J and Meyers C P 1985 Effects of CO2 enrichment and nitrogen stress on growth and partitioning of drymatter and nitrogen in wheat and maize. Aust. J. Plant Physiol. 18, 339-356.
Ingestad T 1982 Relative addition rate and external concentration; Driving variables used in plant nutrition research. Plant Cell Environ. 5, 443-453.
Johnson D W, Ball T and Walker R F 1995 Effects of elevated CO2 and nitrogen on nutrient uptake in ponderosa pine seedlings. Plant Soil 168 169, 535-545.
Kirkby E A and Mengle K 1967 Ionic balance in different tissues of tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 42, 6-14.
Kuehny J S, Peet M M, Nelson P V and Willits D H 1991 Nutrient dilution by starch in CO2-enriched chrysanthemum. J. Exp. Bot. 42, 711-716.
Lambers H, Van Der Werf A and Konings H 1991 Respiratory patterns in roots in relations to their functioning. InPlant Roots: the hidden Half. Eds. Y Waisel, A Eshel and U Kafkafi. pp 229-263. Marcel Dekker Inc, New York.
Lee R B and Rudge K A 1986 Effects of nitrogen deficiency on absorption of nitrate and ammonium by barley plants. Ann. Bot. 57, 471-486.
Montes R A and Christensen N L 1979 Nitrification and succession in the piedmont of North Carolina. For. Sci, 2, 287-297.
Norby R J, Pastor J and Melillo J M 1986 Carbon nitrogen interaction in CO2 enriched white oak: physiological and long-term perspectives. Tree Physiol. 22, 233-241.
Norby R J, O'Neill E G, Hood W G and Luxmore R J 1987 Carbon allocation, root exudation and mycorrhizal colonisation of Pinus echinataseedlings grown under CO2 enrichment. Tree Physiol. 3, 203-210.
Norby R J 1994 Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide. Plant Soil 165, 9-20.
Oliver W W and Ryker R A 1990 Pinus ponderosa Doubl. ex Laws. InSilvics of North America. Volume 1, Conifers. Agriculture Handbook 654. Eds. R M Burns and B H Honkala. United States Dept. of Agriculture, Forest Service, Washington.
Olinger S V, Aber J D, Lovett G M, Millham S E, Lathrop R G and Ellis J M 1994 A spatial model of atmospheric deposition for the northeastern US. Ecol. Appl. 3, 459-472.
Pan W L, Jackson W A and Moll R H 1985 Nitrate uptake and partitioning by corn root systems. Differential effects of ammonium among genotypes and stages of development. Plant Physiol. 77, 560-566.
Pate J S and Layzell D B 1990 Energetic and biological cost of nitrogen assimilation. InBiochemistry of Plants, Vol. 16, Intermediary Nitrogen Metabolism. pp 1-42. Academic Publishing, San Diego.
Poorter H 1993 Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104/105, 77-97.
Rogers H H, Runion G B and Krupa S V 1994 Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollut. 83, 155-189.
Rygiewicz P T and Bledsoe C S 1986 Effects of pretreatment conditions on ammonium and nitrate uptake by Douglas-fir seedlings. Tree Physiol. 1, 145-150.
Schulze E D 1989 Air pollution and forest decline in a spruce (Picea abies) forest. Science 224, 776-783.
Serna M D, Boras R, Legaz F and Primo-Millo 1992 The influence of nitrogen concentration and ammonium/nitrate ratio on N-uptake, mineral composition and yield of citrus. Plant Soil 147, 13-22.
Sokal R R and Rohlf F J 1981 Biometry, 2d ed. WH Freeman, San Francisco.
Thomas R B, Lewis J D and Strain B R 1994 Effects of leaf nutrient status on photosynthetic capacity in loblolly pine (Pinus taeda) seedlings grown in elevated atmospheric CO2. Tree Physiol. 14, 947-960.
Thomas R B, Richter D D, Ye H, Heine P R and Strain B R 1991 Nitrogen dynamics and growth of an N-fixing tree (Gliricidia sepium(Jacq.) Walp.) exposed to elevated atmospheric carbon dioxide. Oecologia 88, 415-421.
Tissue D T, Thomas R B and Strain B R 1993 Long-term effects of elevated C02 and nutrients on photosynthesis and Rubisco in loblolly pine seedlings. Plant Cell Environ. 16, 859-865.
Tissue D T, Thomas R B and Strain B R 1996 Growth and photosynthesis of loblolly pine (Pinus taeda) after exposure to elevated CO2 for 19 months in the field. Tree Physiol. 16, 49-59.
Tolley L C and Strain B R 1984 Effects of CO2 enrichment growth of Liquidambar styracifluaand Pinus taedaseedlings under different irradiance levels. Can. J. For. Res. 14, 343-350.
Tschaplinski T J, Norby R J and Wullschleger D S 1993 Responses of loblolly pine seedlings to elevated CO2 and fluctuating water supply. Tree Physiol. 13, 283-296.
Vitousek P M and Howarth R W 1991 Nitrogen limitation on land and in the sea: How can it occur? Biogeochem. 13, 87-115.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BassiriRad, H., Griffin, K.L., Reynolds, J.F. et al. Changes in root NH +4 and NO −3 absorption rates of loblolly and ponderosa pine in response to CO2 enrichment. Plant and Soil 190, 1–9 (1997). https://doi.org/10.1023/A:1004206624311
Issue Date:
DOI: https://doi.org/10.1023/A:1004206624311