Abstract
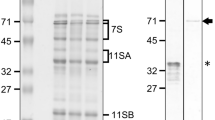
The synthesis and processing of the major storage proteins in soybean cotyledons was studied both in vivo and in vitro. The α and α′ subunits of 7S as well as the 11S proteins are synthesized as higher molecular weight-precursors on membrane-bound polysomes. The initial translation products of the 7S are proteolytically cleaved during translation suggesting the removal of a ‘signal peptide’ as evidenced by the presence of 2α and 2α′ peptides immunoreactive with 7S antibody in the in vitro chain completion products of the membrane-bound polysomes. This is followed or accompanied by cotranslational glycosylation, which increases their size equivalent to that of initially-synthesized precursors. In vivo pulse-labelled 7S α and α′ products are of slightly higher molecular weights than the immunoprecipitable chain-completion products, indicating further post-translational modifications. A slow post-translational processing during a period of 1.5 to 16 h yields the final 7S α and α′ glycoproteins.
Acidic and basic subunits of the 11S protein appear to be synthesized from common large molecular weight (60K-59K) precursors. Antibodies to the 11S acidic component recognize both acidic and basic domains in the precursor while those raised against basic subunits appear to be specific for that region only. The processing of the 11S precursor is also very slow and occurs post-translationally. This slow rate of processing, coupled with a temporal difference in the synthesis of 7S and 11S components, suggests a highly coordinated mechanism for synthesis and packaging of these proteins into protein bodies during seed development.
Similar content being viewed by others
References
Bailey DS, De Luca V, Verma DPS and Maclachlan GA (1980) Involvement of lipid-linked oligosaccharides in the synthesis of storage glycoproteins in soybean seeds. Plant Physiol 66: 1113–1118.
Baulcombe D and Verma DPS (1978) Preparation of a complementary DNA for leghaemoglobin and direct demonstration that leghaemoglobin is encoded by the soybean genome. Nucl Acid Res 5: 4141–4153.
Beachy RN, Thompson JF and Madison JT (1978) Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol 61: 139–144.
Beachy RN, Thompson JF and Madison JT (1979) Isolation and characterization of messenger RNAs that code for the subunits of soybean seed protein. The Plant Seed: Development, Preservation and Germination. eds. I Rubenstein, RL Phillips, CE Green and BG Gengenbach. Academic Press, New York, pp. 67–84.
Bielinska M and Boime I (1979) Glycosylation of human chorionic gonadotropin in mRNA-dependent cell-free extracts: Post-translational processing of an asparagine-linked mannose-rich oligosaccharide. Proc Natl Acad Sci USA 76: 1208–1212.
Blobel G and Dobberstein B (1975) Transfer of proteins across membranes. Presence of proteolytically processed and unprocessed nascent immunoglobin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol 67: 835–851.
Bollini R and Chrispeels MJ (1979) The rough endoplasmic reticulum is the site of reserve protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146: 487–501.
Burr B and Burr FA (1976) Zein synthesis in maize endosperm on polyribosomes attached to protein bodies. Proc Natl Acad Sci USA 73: 515–519.
Croy RRD, Gatehouse JA, Evans IM and Boulter D (1980) Characterization of the storage protein subunits synthesized in vitro by polyribosomes and RNA from developing pea (Pisum sativum L.). Planta 148: 49–56.
Gagnon J, Palmiter RD and Walsh KA (1978) Comparison of the NH2-terminal sequence of ovalbumin synthesized in vitro and in vivo. J. Biol. Chem. 253, 7464–7468.
Hill JE and Breidenbach RW (1974) Proteins of soybean seeds. II. Accumulation of the major protein components during seed development and maturation. Plant Physiol 53: 742–746.
Hunt LA, Etchison JR and Summers DF (1978) Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitus virus. Proc Natl Acad USA 75: 754–758.
James DW and Elbein AD (1980) Effects of several Tunicamycin-like antibiotics on glycoprotein biosynthesis in mung beans and suspension cultured soybean cells. Plant Physiol 65: 460–464.
Knipe DM, Baltimore D and Lodish HF (1977) Separate pathways of maturation of the major structural proteins of vesicular stomatitus virus. J Virol 21: 1128–1139.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Laskey RA and Mills AD (1975) Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem 56: 335–341.
Lingappa VR, Lingappa JR, Prasad R, Ebner KE and Blobel G (1978) Coupled cell-free synthesis, segregation and core-glycosylation of a secretory protein. Proc Natl Acad Sci USA 75: 2338–2342.
Marcu K and Dudock B (1974) Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nuc Acid Res 1: 1385–1397.
Moreira MA, Hermodson MA, Larkins BA and Nielson NC (1979) Partial characterisation of the acidic and basic polypeptides of glycinin. J Biol Chem 254: 9921–9926.
Northcote D.H. (1979) The involvement of the Golgi apparatus in the biosynthesis and secretion of glycoproteins and polysaccharides. Biomembranes 10: 51–75.
Rothman JE and Lodish HF (1977) Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature 269: 775–780.
Sun SM, Buchbinder BU and Hall TC (1975) Cell-free synthesis of the major storage protein of the bean Phaseolus vulgaris L. Plant Physiol 56: 780–785.
Thanh VH and Shibasaki R (1976) Heterogeneity of beta-conglycinin. Biochim Biophys Acta 439: 326–338.
Toneguzzo F and Ghosh HP (1977) Synthesis and glycosylation in vitro of glycoprotein of vesicular stomatitis virus. Proc Nat Acad Sci USA 74: 1516–1520.
Towbin H, Staehelin T and Gorden J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Verma DPS, Nash DT and Schulman H (1974) Isolation and in vitro translation of leghaemoglobin messenger RNA in soybean. Nature 251: 74–77.
Verma DPS and Maclachlan GA (1976) Metabolism of Poly(A) in plant cells. Discrete classes associated with free and membrane-bound polysomes. Plant Physiol 58: 405–410.
Verma DPS, Ball S, Guerin CW and Wanamaker L (1979) Leghaemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghaemoglobins. Biochem 18: 476–483.
Wilden WVD, Herman EM and Chrispeels MJ (1980) Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci USA 77: 428–432.
Yamanchi F, Thanh VH, Kawase M and Sibasaki K (1976) Separation of the glycopeptides from soybean 7S protein: their amino acid sequence. Agr Biol Chem 40: 691–696.
Zagbury D, Uhr JW, Jamieson JD and Palade GE (1970) Immunoglobin synthesis and secretion. II. Radioautographic studies of intracellular transport and sites of addition of the carbohydrate moities. J Cell Biol 46: 52–63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sengupta, C., Deluca, V., Bailey, D.S. et al. Post-translational processing of 7S and 11S components of soybean storage proteins. Plant Mol Biol 1, 19–34 (1981). https://doi.org/10.1007/BF00023011
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00023011