Abstract
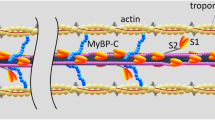
A ’freeze break‘ technique and immunoelectronmicroscopy were used to study the elastic properties of cardiactitin filaments. Small bundles consisting of a few fibres fromfreshly prepared dog papillary muscle were quickly frozen andbroken under liquid nitrogen to fracture sarcomeres in planesperpendicular to the filament axes. Breaks occurred at each ofseveral regions along the sarcomeres. The still-frozen specimenswere thawed during fixation to allow elastic filaments toretract. The broken muscle segments were then treated withmonoclonal titin antibody 9D10 which labelled a unique epitope inthe I-band. In sarcomeres broken at the A-I junction, the titinfilaments reacted toward the Z-line, independently of the thinfilaments. The retracted epitopes did not reach the Z-line;retraction stopped at the N1-line level. In sarcomeres brokennear the Z-line, the titin filaments retracted in the oppositedirection, to the tip of the thick filaments. When the breakoccurred in the A-band, by contrast, the titin-epitope positionwas unaffected. On the basis of these results, and despite thereported interaction of titin and actin in vitro, it appears thatcardiac titin molecules form elastic filaments that arefunctionally independent of the thin filaments. Near the Z-line,however, the titin filaments seem to associate firmly with thethin filaments
Similar content being viewed by others
References
FUNATSU, T., KONO, E., HIGUCHI, H., KIMURA, S., ISHIWATA, S., YOSHIOKA, T., MARUYAMA, K., & TSUKITA, S. (1993) Elastic filaments in situ in cardiac muscle: deep-etch replica analysis in combination with selective removal of actin and myosin filaments. J. Cell Biol. 120, 711–24.
FÜRST, D., OSBORN, M., NAVE, R. & WEBER, K. (1988) The organisation of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z-line extends close to the M-line. J. Cell Biol. 106, 1563–72.
FÜRST, D., OSBORN, M. & WEBER, K. (1989) Repetitive titin epitopes with a 42 nm spacing coincide in relative position with known A-band striation also identified by major associated proteins. J. Cell Sci. 194, 119–25.
GRANZIER, H., HELMES, M. & TROMBITAS, K. (1996) Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophysical J. 70, 430–42.
HELMES, M., TROMBITÁS, K. & GRANZIER, H. L. (1995) Titin develops restoring force in rat cardiac myocytes. Circulation Res. 79, 620–7.
ITOH, Y., SUZUKI, T., KIMURA, S., OHASHI, K., HIGUCHI, H., SAWADA, H., SHIMIZU, T., SHIBATA, M. & MARUYAMA, K. (1988) Extensible and less-extensible domains of connectin filaments in stretched vertebrate skeletal muscle as detected by immunofluorescence and immunoelectron microscopy using monoclonal antibodies. J. Biochem. 104, 504–8.
JIN, J. P. (1995) Cloned rat cardiac titin class I and class II motifs: expression, purification, characterisation, and interaction with F-actin. J. Mol. Chem. 270, 6908–16.
KELLERMAYER, M. & GRANZIER, H. (1996a) Calcium-dependent inhibition of in vitrothin-filament motility by native titin. Biophysical J. 70, A269.
KELLERMAYER, M. & GRANZIER, H. L. (1996b) Elastic properties of single titin molecules made visible through fluorescent F-actin binding. Biochem. Biophys. Res. Commun. 221, 491–7.
LABEIT, S. & KOLMERER, B. (1995) Titin: giant protein in charge of muscle ultrastructure and elasticity. Science 270, 293–6.
LI, Q., JIN, J. P. & GRANZIER, H. (1995) The effect of genetically expressed cardiac titin fragments on in vitroactin motility. Biophysical J. 69, 1508–18.
LINKE, W. A., IVEMEYER, M., OLIVIERI, N., KOLMERER, B., RÜEGG, G. & LABEIT, S. (1996) Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 261, 62–71.
MARUYAMA, K. (1994) Connectin, an elastic protein of striated muscle. Biophys. Chem. 50, 73–85.
MARUYAMA, K., HU, D. H. & SUZUKI, T. (1987) Binding to actin filaments to connectin. J Biochem. 101, 1339–46.
PAN, K-M., DAMODARAN, S. & GREASER, M. L. (1994) Isolation and characterization of titin T1 from bovine cardiac muscle. Biochem. 33, 8255–61.
REEDY, M. K. & REEDY, M. C. (1985) Rigor cross-bridge structure in tilted single filament layer and flared-x formations from insect flight muscle. J. Mol. Biol. 185, 145–76.
TRINICK, J. (1991) Elastic filaments and giant proteins in muscle. Curr. Opin. Cell Biol. 3, 112–18.
TRINICK, J. (1996) Titin as a scaffold and spring. Curr. Biol. 6, 258–60.
TROMBITÁS, K. (1971) The submicroscopic transversal structure of striated fibril. Acta Biochim. Biophys. 6, 419–27.
TROMBITÁS, K. & POLLACK, G. H. (1993a) Elastic properties of the titin filaments in the Z-line region of vertebrate striated muscle. J. Muscle Res. Cell Motil. 14, 416–22.
TROMBITÁS, K. & POLLACK, G. H. (1993b) Elastic properties of connecting filaments along sarcomere. In Mechanism of Myofilament Sliding in Muscle Contraction. (edited by SUGI, H. & POLLACK, G. H.) pp. 71–9. New York: Plenum Press.
TROMBITÁS, K., BAATSEN, P., KELLERMAYER, M. & POLLACK, G. H. (1991) Nature and origin of gap filaments in striated muscle. J. Cell. Sci. 100, 809–14.
TROMBITÁS, K., POLLACK, G. H., WRIGHT, J. & WANG, K. (1993) Elastic properties of titin filaments demonstrated using a ‘freeze-break’ technique. Cell. Motil. Cytoskeleton. 24, 274–83.
TROMBITÁS, K., JIN, J. P. & GRANZIER, H. (1995a) The mechanically active domain of titin in cardiac muscle. Circular Res. 77, 856–61.
TROMBITÁS, K., GREASER, M. L. & POLLACK, G. H. (1995b) Interaction between cardiac titin and actin? Biophys J. 68, A64.
WANG, K. (1985) Sarcomere associated cytoskeletal lattice in striated muscle In Cell and Muscle Motility (edited by SHAY, J. W.) pp. 315–69. New York: Plenum Press.
WANG, S. M. & GREASER, M. L. (1985) Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J. Muscle Res. Cell Motil. 6, 293–312.
WANG, K., MCARTER, R., WRIGHT, J., JENNATE, B. & RAMIREZ-MITHCELL, R. (1991) Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc. Natl Acad. Sci. USA. 88, 7101–5.
WANG, K., MCARTER, R., WRIGHT, J., JENNATE, B. & RAMIREZ-MITCHELL, R. (1993) Viscoelasticity of the sarcomere matrix of skeletal muscle: the titin-myosin composite filament is a dual-range molecular spring. Biophys J. 64, 1161–77.
WHITING A., WARDALE, J. & TRINICK, J. (1988) Does titin regulate the length of muscle thick filaments? J. Mol. Biol. 205, 263–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TROMBITAS, K., GREASER, M.L. & POLLACK, G.H. Interaction between titin and thin filaments in intact cardiac muscle. J Muscle Res Cell Motil 18, 345–351 (1997). https://doi.org/10.1023/A:1018626210300
Issue Date:
DOI: https://doi.org/10.1023/A:1018626210300