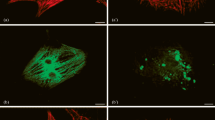
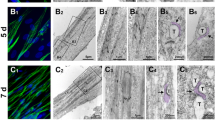
Summary
Established myogenic cell lines of different species and tissue origin have been used to study expression and organisation of muscle-specific proteins during differentiation. Furthermore, primary cultures of rat myocard cells were used to examine these same processes during dedifferentiation. In particular, we were interested in the general mechanism that underlies the changes in the supramolecular organisation of titin during in vitro myogenesis. It became obvious that in the differentiating muscle cell cultures the redistribution of desmin, actin and myosin in a typical, differentiation state dependent fashion, always showed a certain delay when compared to titin. The sequence of changes in the assembly of cytoskeletal and sarcomeric structures observed during differentiation of the cell lines was reversed during the process of dedifferentiation in cultured rat myocard cells. These results all indicate that titin is an early marker of myogenic differentiation, both in vivo and in vitro, and that the typical reorganisation of this giant molecule is independent of species or muscle cell type.
Similar content being viewed by others
References
AUSMAJ., FÜRSTD., THONÉF., FLAMENGW., WEBERK., RAMAEKERSF. & BORGERSM. (1995) Molecular changes of titin in left ventricular dysfunction as a result of chronic hibernation. J. Mol. Cell. Cardiol. 27, 1203–12.
BABAÏF., MUSEVI-AGHDAMJ., SCHÜRCHW., ROYALA. & GABBIANIG. (1990) Coexpression of α-sarcomeric actin, α-smooth muscle actin and desmin during myogenesis in rat and mouse embryos. I. Skeletal muscle. Differentiation 44, 132–42.
BADERD., MASAKIT. & FISCHMAND. A. (1982) Immunohistochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 95, 763–70.
BAINSW., PONTEP., BLAUH. & KEDESL. (1984) Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol. Cell. Biol. 4, 1449–53.
BARBETJ. P., THORNELLL.-E. & BUTLER-BROWNG. S. (1991) Immunocytochemical characterisation of two generations of fibers during the development of the human quadriceps muscle. Mech. Dev. 53, 3–11.
BLAUH., CHIUC.-P. & WEBSTERC. (1983) Nucleoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–80.
BOCHATON-PIALLATM.-L., ROPRAZP., GABBIANIG., SANTEUSANIOG., PALMEIRIG., SCHIAROLIS. & SPAGNOLIL. G. (1992) Actin isoforms and intermediate filament protein expression in human developing skeletal muscle. B.A.M. 2, 83–7.
BUCKINGHAMM. E. (1985) Actin and myosin multigene families: their expression during the formation of skeletal muscle. Essays Biochem. 20, 77–109.
DEBUSW., WEBERK. & OSBORNM. (1983) Monoclonal antibodies to desmin, the muscle specific intermediate filament. EMBO J. 2, 2305–12.
DEGROOTI. J. M., LAMERSW. H. & MOORMANA. F. M. (1989) Isomyosin expression patterns during rat heart morphogenesis: an immuno-histochemical study. Anat. Rec. 224, 365–73.
DeJONGF., GEERTSW. J. C., LAMERSW. H., LOSJ. A. & MOORMANA. H. M. (1990) Isomyosin expression during formation of the tubular chicken heart: a three-dimensional immunohistochemical analysis. Anat. Rec. 226, 213–27.
DLUGOSZA. A., ANTINP. B., NACHMIASV. T. & HOLTZERH. (1984) The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J. Cell Biol. 99, 2268–78.
FISCHMAND. A. (1986) Myofibrillogenesis and the morphogenesis of skeletal muscle. In Myology. Basic and Clinical. (edited by ENGELA. G. & BANKERB. Q.) pp. 5–30. New York: McGraw-Hill.
FRANKE. D., TUSZYNSKIP. & WARRENL. (1982) Localization of vimentin and desmin in BHK21/C13 cells and in baby hamster kidney. Exp. Cell Res. 139, 235–47.
FULTONA. B. & ISAACSW. B. (1991) Titin, a huge, elastic sarcomeric protein with a probable role in morphogenesis. BioEssays 13, 157–61.
FÜRSTD. O., OSBORNM., NAVER. & WEBERK. (1989) Myogenesis in the mouse embryo: differential onset of expression of myogenic proteins and the involvement of titin in myofibril assembly. J. Cell Biol. 109, 517–27.
GARDD. & LAZARIDESE. (1980) The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 19, 263–75.
GREASERM. L., HANDELS. E., WANGS.-M., SCHULTZE., BULINSKIJ. C. & LESSARDJ. L. (1989) Assembly of titin, myosin, actin and tropomyosin into myofibrils in cultured chick cardiomyocytes. In Cellular and molecular biology of muscle development, New Series, Vol. 93. (edited by STOCKDALEF. & KEDESL.) pp. 246–57. New York: Liss.
HANDELS. E., WANGS.-M., GREASERM., SCHULTZE., BULINSKIJ. C. & LESSARDJ. L. (1989) Skeletal muscle myofibrillogenesis as revealed with a monoclonal antibody to titin in combination with detection of the alpha-and gamma isoforms of actin. Dev. Biol. 132, 35–44.
HANDELS. E., GREASERM. L., SCHULTZE., WANGS.-M., BULINSKIJ. C., LINJ. J.-C. & LESSARDJ. L. (1991) Chicken cardiac myofibrillogenesis studied with antibodies specific for titin and the muscle and nonmuscle isoforms of actin and tropomyosin. Cell Tissue Res. 263, 419–30.
HOLTZERH., SCHULTHEISST., DILULLOC., CHOIJ., COSTAM., LUM. & HOLTZERS. (1990) Autonomous expression of the differentiation programs of cells in the cardiac and skeletal myogenic lineages. Ann. NY Acad. Sci. 599, 158–69.
KIMESB. W. & BRANDTB. L. (1976) Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 98, 367–81.
LAEMMLIU. K. (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227, 680–5.
LOMPRÉA.-M., NADAL-GINARDB. & MAHDAVIV. (1984) Expression of the cardiac ventricular α-and β-Myosin Heavy Chain genes is developmentally and hormonally regulated. J. Biol. Chem. 259, 6437–46.
LUM. H., DILULLOC., SCHULTHEISST., HOLTZERS., MURRAYJ. M., CHOIJ., FISCHMANND. A. & HOLTZERH. (1992) The vinculin/sarcomeric α-actinin/α-actin nexus in cultured cardiac myocytes. J. Cell Biol. 117, 1007–22.
LYONSG., SCHIAFFINOS., SASSOOND., BARTONP. & BUCKINGHAMM. (1990) Development regulation of myosin gene expression in mouse cardiac muscle. J. Cell Biol. 111, 2427–36.
MARUYAMAK. (1994) Connectin, an elastic protein of striated muscle. Biophysical Chemistry 50, 73–85.
MASAKIT., BADERD. M., REINACHF. C., SHIMIZUT., OBINATAT., SHAFIQS. A. & FISCHMAND. A. (1982) Monoclonal antibody analysis of myosin heavy chain and the protein isoforms during myogenesis. In Molecular and cellular control of muscle development. (edited by PEARSONM., EPSTEINH., KAUFMANH. S. & GARRELSJ. L.) pp. 405–17. New York: Cold Spring Harbor Lab Press.
NAGA. C., KREHELW. & CHENGM. (1986) Distribution of vimentin and desmin filaments in embryonic cardiac muscle cells in culture. Cytobios 45, 195–209.
OSBORNM. & WEBERK. (1982) Immunofluorescence and immunocytochemical procedure with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol. 24, 97–132.
PIEPERF. R., SLOBBER., RAMAEKERSF. C. S., CUIJPERSH. T. & BLOEMENDALH. (1987) Upstream regions of the hamster desmin and vimentin genes regulate expression during in vitro myogenesis. EMBO J. 6, 3611–18.
QUINLANR. A. & FRANKEW. W. (1982) Heteropolymer of vimentin and desmin in vascular smooth muscle and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc. Natl. Acad. Sci. USA 79, 3452–6.
RAMAEKERSF. C. S., HUIJSMANSA., MOESKERO., KANTA., JAPP., HERMANC. & VOOIJSP. (1983) Monoclonal antibodies against keratin filaments, specific for glandular epithelia and their tumours. Use in surgical pathology. Lab. Invest. 49, 353–61.
RAMAEKERSF. C. S., MOESKERO., HUIJSMANSA., SCHAARTG., WESTERHOFG., WAGENAAR, HERMANC. J. & VOOIJG. P. (1985) Intermediate filament proteins in the study of tumor heterogeneity: an indepth study of tumors of the urinary and respiratory tracts. Ann. NY Acad. Sci. 455, 614–34.
RAMAEKERSF. C. S., HUIJSMANSA., SCHAARTG., MOESKERO. & VOOIJSG. P. (1987) Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Expl. Cell Res 170, 235–49.
RUDZICKAD. L. & SCHWARZR. J. (1988) Sequential activation of α-actin genes during avian cardiogenesis: vascular smooth muscle α-actin gene transcripts mark the onset of cardiomyocyte differentiation. J. Cell Biol. 107, 2575–86.
SCHAARTG., VIEBAHNC., LANGMANNW. & RAMAEKERSF. C. S. (1989) Desmin and titin expression in early postimplantation mouse embryos. Development 107, 585–96.
SCHAARTG., PIEPERF. R., KUIJPERSH. J. H., BLOEMENDALH. & RAMAEKERSF. C. S. (1991) Baby hamster kidney (BHK-21/C13) cells can express striated muscle type proteins. Differentiation 46, 105–15.
SCHAARTG., VAN DERVENP. F. M. & RAMAEKERSF. C. S. (1993) Characterization of cardiotin, a structural component in the myocard. Eur. J. Cell Biol. 62, 34–48.
SKALLIO., ROPRAZP., TRZECIAKA., BENZONANAG., GILLESSEND. & GABBIANIG. (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J. Cell Biol. 103, 2787–96.
SKALLIO., GABBIANIG., BABAÏF., SEEMAYERT. A., PIZZOLATOG. & SCHÜRCHW. (1988) Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation and origin. II. Rhabdomyosarcomas. Am. J. Pathol. 130, 513–31.
STOKERM. P. G. & MACPHERSONI. A. (1961) Studies on transfection of hamster cells by polyoma virus in vitro. Virology 14, 359–70.
TOKUYASUK. T. & MAHERP. A. (1987) Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. I. Presence of immunofluorescent titin spots in premyofibril stages. J. Cell Biol. 105, 2781–93.
TOKUYASUK. T., MAHERP. A. & SINGERS. J. (1984) Distributions of vimentin and desmin in developing chick myotubes in vivo. I. Immunofluorescence study. J. Cell Biol. 96, 1961–72.
TOWBINH., STAEHELINT. & GORDONJ. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–3.
TRAUBP. (1985) Intermediate filaments: a review. Berlin: Springer Verlag.
TRINICKJ. (1992) Understanding the functions of titin and nebulin. FEBS Lett. 307, 44–8.
VANDEKERCKHOVEJ. & WEBERK. (1978) At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 126, 783–802.
VANDEKERCKHOVEJ. & WEBERK. (1981) Actin typing on total cellular extracts: a highly sensitive proteinchemical procedure able to distinguish different actins. Eur. J. Biochem. 113, 595–603.
VANDEKERCKHOVEJ., BUGAISKYG. & BUCKINGHAMM. (1986) Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. J. Biol. Chem. 261, 121–9.
VAN DERLOOPF. T. L., SCHAARTG., LANGMANNW., RAMAEKERSF. C. S. & VIEBAHNCH. (1992) Expression and organization of muscle specific proteins during the early developmental stages of the rabbit heart. Anat. Embryol. 185, 439–50.
VAN DERVENP. F. M., SCHAARTG., JAPP. H. K., SENGERSR. C. A., STADHOUDERSA. M. & RAMAEKERSF. C. S. (1992) Differentiation of human skeletal muscle cells in culture: maturation as indicated by titin and desmin striation. Cell Tissue Res. 270, 189–98.
VAN DERVENP. F. M., SCHAARTG., CROESH. J. S., JAPP. H. K., GINSELL. A. & RAMAEKERSF. C. S. (1983) Titin aggregates associated with intermediate filaments align along stress fiber-like structures during human skeletal muscle cell differentiation. J. Cell Sci. 106, 749–59.
VIEBAHNCH., LANEE. B. & RAMAEKERSF. C. S. (1988) Keratin and vimentin expression in early organogenesis in the rabbit embryo. Cell Tissue Res. 253, 553–62.
VITADELLOM., MATTEOLIM. & GORZAL. (1990) Neurofilament proteins are co-expressed with desmin in heart conduction system myocytes. J. Cell Sci. 97, 11–21.
WANGS.-M. & GREASERM. L. (1985) Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J. Muscle Res. Cell Motil. 6, 293–312.
WHALENR. G., SELLS. M., BUTLER-BROWNG. S., SCHWARTZK., BOUVERETP. & PINSET-HÄRSTRÖMI. (1981) Three myosin heavy chain isozymes appear sequentially in rat muscle development. Nature 292, 805–9.
WOODCOCK-MITCHELLJ., MITCHELLJ. J., LOWR. B., KIENYM., SENGELP., RUBBIAL., SKALLIO., JACKSONB. & GABBIANIG. (1988) α-Smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles. Differentiation 39, 161–6.
YAFFED. & SAXELO. (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van der Loop, F.T.L., van Eys, G.J.J.M., Schaart, G. et al. Titin expression as an early indication of heart and skeletal muscle differentiation in vitro. Developmental re-organisation in relation to cytoskeletal constituents. J Muscle Res Cell Motil 17, 23–36 (1996). https://doi.org/10.1007/BF00140321
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00140321