Abstract
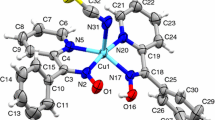
Complexes of the type M(Pa)2(HAz)2 and M(QA)2(HAz)2 (M=cobalt(II) and nickel(II); HPa=picolinic acid, HQa=quinaldic acid; HAz=azoles like imidazole (Him), pyrazole (HPz), benzimidazole (HBzIm) etc.) show a similar thermal behaviour. In the first step of decomposition the corresponding azolinium picolinates or quinaldinates (H2AzPa, H2AzQa) are split off with formation of polymeric mixed ligand complexes M(Pa)(Az) or M(Qa)(Az). X-ray analysis of Co(Qa)2(HBzIm)2 XIIIa illustrates a proton transfer and a subsequent thermal removal of benzimidazolinium quinaldinate (H2BzImQa): Hydrogen bridges from pyrrole nitrogen of the benzimidazole to the non-coordinated oxygen of the quinaldinate predetermine the thermal initiated proton transfer. The high volatility of the heterocyclic acids and the nitrogen coordination are responsible for the formation of the mixed ligand complex Co(Qa)(BzIm) XIVa.
Exceptions are the complexes M(Pa)2(HPz)2 XIa-b and M(Qa)2(HIm)2 XVIIa-b. Pyrazole is eliminated from the complexes XIa-b with formation of the solvent-free inner complex M(Pa)2 XIIa-b. From compounds XVIIIa-b quinaldic acid or their decomposition products are split off and a high temperature modification of M(Im)2 XVIIIa-b is formed at elevated temperature. XVIIIa-b are decomposed to the cyanides M(CN)2 similarly to the thermal behaviour of Cu(Im).
In the first step the thermal degradation of imidazole and pyrazole adducts of copper(II) picolinates and quinaldinates is characterized by the elimination of azoles. The reason for this thermal behaviour is the weaker coordination of the azole heterocycles in copper chelate compounds.
Similar content being viewed by others
References
R. Österberg, Coord. Chem. Rev., 12 (1974) 309.
R. G. Bhirud and T. S. Srivastava, Inorg. Chim. Acta, 173 (1990) 121.
A. Dobry-Duclaux and A. May, Bull. Soc. Chim. Biol., 52 (1970) 1447.
K. Yamaguchi and D. T. Sawyer, Inorg. Chem., 24 (1985) 971.
T. Matsushita, L. Spencer and D. T. Sawyer, Inorg. Chem., 27 (1988) 1167.
E. Libby, R. J. Webb, W. E. Streib, K. Folting, J. C. Huffman, D. N. Hendrickson and G. Christou, Inorg. Chem., 28 (1989) 4037.
S. C. Chang, J. K. H. Ma, J. T. Wang and N. C. Li, J. Coord. Chem., 2 (1972) 31.
H. Loiseleur, G. Thomas, B. Cheverier and D. Grandjean, J. Chem. Soc. Chem. Commun., (1967) 182.
A. Takenaka, H. Utsumi, N. Ishihara, A. Furusaki and I. Nitta, Nippon Kagaku Zasshi, 91 (1970) 921.
R. D. Gillard, S. H. Laurie and F. S. Stephens, J. Chem. Soc., A (1968) 2588.
A. Takenaka, H. Utsumi, T. Yamamoto, A Furusaki and I. Nitta, Nippon Kagaku Zassi, 91 (1970) 928.
A. Böttcher, M. Döring, E. Uhlig, M. Fedtke, K. Dathe and B. Nestler, PCT-Patent 1991, WO 91/13925 from 19.09.1991.
M. Döring, W. Ludwig, M. Meinert and E. Uhlig, Z. anorg. allg. Chem., 595 (1991) 45.
M. Döring, W. Ludwig, E. Uhlig, S. Wocadlo and U. Müller, Z. anorg. allg. Chem., 611 (1992) 61.
W. Ludwig, M. Döring, R. Fischer, A. Friedrich, W. Seider, E. Uhlig and D. Walther, J. Thermal Anal., 42 (1994) 443.
M. Döring, W. Ludwig and H. Görls, J. Thermal Anal., 42 (1994) 443.
W. Ludwig, J. Thermal Anal., 8 (1975) 75.
MOLEN, An Interactive Structure Solution Procedure, Enraf-Nonius, Delft, The Netherlands 1990.
G. M. Sheldrick: SHELXS, Program for Crystal Structure Solution, Göttingen 1980.
M. Döring, Habilitation Thesis, University Jena 1994.
A. Gadet, Acta Crystallogr., B30 (1974) 349.
H. L. Henrikson, Acta Crystallogr., B33 (1977) 1947.
S. J. Ashcroft and C. T. Mortimer, ‘Thermochemistry of Transition Metal Complexes’, Academic Press, New York 1970.
M. Döring and H. Görls, unpublished results.
H. A. Staab and W. Rohr, Newer Methods of Prep. Org. Chem., 6 (1967) 61C.
I. B. Bersucker, Coord. Chem. Rev., 14 (1974) 357.
Sigwart, P. Kroneck and P. Hemmerich, Helv. Chim. Acta, 53 (1970) 177.
H. Irving and R. J. P. Wiliams, J. Chem. Soc. (1953) 3192.
R. Lehnert and F. Seel, Z. anorg. allg. Chem., 444 (1978) 91.
I. Bertini, L. Banci, M. Piccioli and C. Luchinat, Coord. Chem. Rev., 100 (1990) 67.
Author information
Authors and Affiliations
Additional information
For Part I see Ref. [8].
We are grateful to the Fonds der Chemischen Industrie for financial support.
Rights and permissions
About this article
Cite this article
Döring, M., Wuckelt, J., Ludwig, W. et al. Thermal latent coordination compounds. Journal of Thermal Analysis 50, 569–586 (1997). https://doi.org/10.1007/BF01979029
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01979029