Abstract
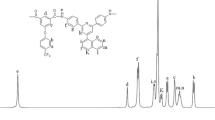
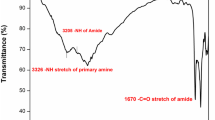
Aromatic polyamides containing different numbers of p-oxyphenylene groups and different catenated positions in the benzene rings were prepared from terephthalic acid (TPA) and isophthalic acid (IPA) with various aryloxy-containing diamines by means of the phosphorylation polycondensation reaction. Most of the polyamides were moderately to highly crystalline, as indicated by X-ray diffraction and DSC measurements. Polyisophthalamides were readily soluble in polar amide-type solvents such as N-methyl-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMAc). Some non-crystalline polyamides could afford tough films by solution casting. Most polyisophthalamides revealed discernible glass transition on their DSC curves, and their Tg values were recorded in the range of 215–238 °C. No discernible glass transitions were observed for the polyamides of TPA by DSC. The thermal stability of these polymers did not show clear dependence on the structure of the diacid or the diamine. In addition, a series of polyamides having pendant groups was synthesized from the polycondensation of TPA and IPA with 1,4-bis(4-aminophenoxy)benzene and its derivatives with methyl, tert-butyl, or phenyl substituent on the central benzene ring. In most cases, the incorporation of pendant groups generally resulted in an enhanced solubility and a decreased Tg and crystallinity.
Similar content being viewed by others
References
T. I. Bair, P. W. Morgan and F. L. Killiam, Macromolecules, 10, 1396 (1977).
M. Panar and L. F. Beste, Macromolecules, 10, 1401 (1977).
H. H. Yang, Aromatic High-Strength Fibers, Wiley; New York, 1989, pp. 66–289.
Kevlar Aramid; Du Pont Product Bulletin.
G. Maglio, R. Palumbo and M. C. Vignola, Macromol. Chem. Phys., 196, 2775 (1995).
S.-H. Hsiao and C.-F. Chang, Macromol. Chem. Phys., 197, 1255 (1996).
C.-P. Yang and W.-T. Chen, Macromol. Chem., 194, 1595 (1993).
C.-P. Yang and J.-J. Cheng, J. Polym. Sci. Polym. Chem., 33, 2209 (1995).
S.-H. Hsiao and K.-Y. Chu, J. Polym. Sci. Polym. Chem., 35, 3385 (1997).
F. Akutsu, M. Inoki, K. Sunouchi, Y. Sugama, Y. Kasashima, K. Naruchi and M. Miura, Polymer, 39, 1637 (1998).
S.-H. Hsiao, C.-P. Yang, M.-H. Chuang and S.-J. Lin, J. Polym. Sci. Polym. Chem., 37, 4510 (1999).
D.-J. Liaw, B.-Y. Liaw and J.-J. Chen, J. Polym. Sci. Polym. Chem., 38, 797 (2000).
J. F. Espeso, J. G. de la Campa, A. E. Lozano and J. de Abajo, J. Polym. Sci. Polym. Chem., 38, 104 (2000).
S.-H. Hsiao, C.-P. Yang and C.-K. Lin, J. Polym. Res., 2, 1 (1995).
S. Tamai, A. Yamaguchi and M. Ohta, Polymer, 37, 3683 (1996).
N. Yamazaki, F. Higashi and J. Kawabata, J. Polym. Sci., Polym. Chem. Ed., 12, 2149 (1974).
N. Yamazaki, M. Matsumoto and F. Higashi, J. Polym. Sci., Polym. Chem. Ed., 13, 1373 (1975).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiao, SH., Chen, YJ. Polyamides based on diamines containing aryloxy groups: Structure-property relationships. J Polym Res 7, 205–213 (2000). https://doi.org/10.1007/s10965-006-0121-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10965-006-0121-0